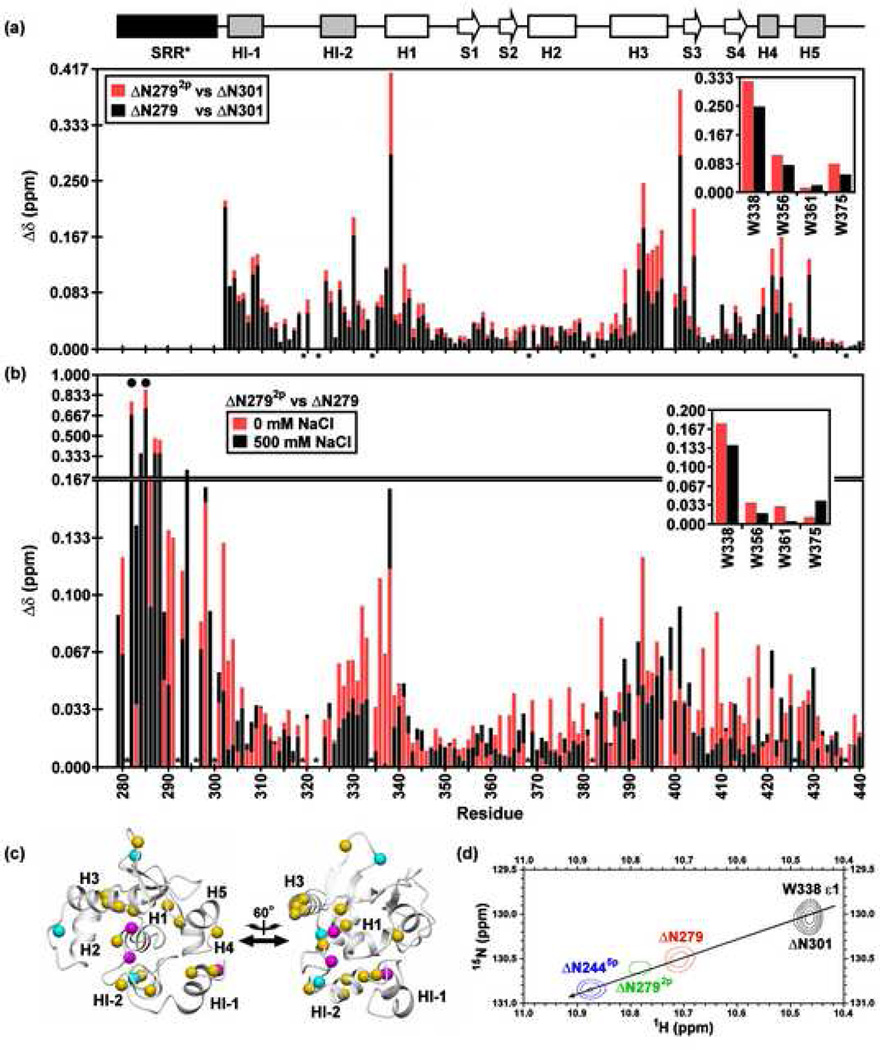
Fig. 6.
Amide 1HN and 15N chemical shift perturbations of the inhibitory module and DNA binding interface by the SRR* are enhanced by phosphorylation and partially reduced with increasing ionic strength. Shown are the combined backbone amide and tryptophan indole (insert) chemical shift perturbations (Δδ) for corresponding residues in (a) ΔN279 (black) and ΔN2792p (red) versus ΔN301 at 500 mM NaCl, and (b) ΔN2792p versus ΔN279 in 0 mM (red) and 500 mM (black) NaCl. A difference plot of the data in (b) is provided as Supplemental Fig. S5. The histogram bar for the smaller change is shown in front of that for the larger change. The absence of a bar indicates that the Δδ could not be unambiguously measured for a given residue due to spectral overlap or weak signals in at least one species or condition. Dark circles and asterisks represent the phosphoacceptor serines and prolines, respectively. (c) As expected, S282 and S285 underwent the largest chemical shift changes upon phosphorylation, with smaller effects occurring for adjacent amides within the SRR*. However, spectral perturbations also occurred in the inhibitory module, H1, and the DNA binding interface, as shown by mapping the amide shift difference between ΔN2792p and ΔN279 at 0 mM NaCl onto the ΔN301 structure (magenta, Δδ > 0.15 ppm; cyan, 0.10 to 0.15 ppm; yellow, 0.05 to 0.10 ppm). The two views are rotated by 60° about the vertical axis. (d) A co-linear relationship is observed between chemical shift and increasing autoinhibition, indicating an allosteric shift in the conformational equilibrium of the regulatable core6. This is illustrated by an overlay of the sidechain indole signals of W338 in four constructs at 500 mM NaCl (ΔN301, black; ΔN279, red; ΔN2792p, green; ΔN2445p, blue). ΔN301 is poorly soluble in low ionic strength buffers, thus precluding comparisons at reduced salt concentrations.