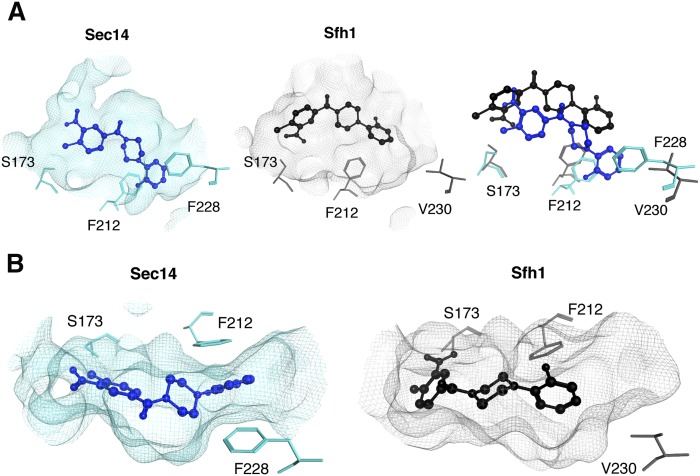
Fig. 1.
Comparisons of NPPM 6748-481 docking poses in the hydrophobic pockets of Sec14 and Sfh1. A: Modeled NPPM 6748-481 poses within the hydrophobic pockets of Sec14 (left panel, blue) and Sfh1 (middle panel, black) are shown. Residues of interest are labeled. The right panel shows a superposition of the binding modes in the two distinct pocket environments and highlights the differences between the two binding modes. Sec14 residue F228 is projected to stabilize NPPM binding via stacking interactions, and these interactions are not available in the Sfh1 context. B: Top views of NPPM-481 docked to Sec14 (left panel) and Sfh1 (right panel) are shown. The protein pocket is rendered in surface mesh with residues of interest highlighted in ball and stick representation.