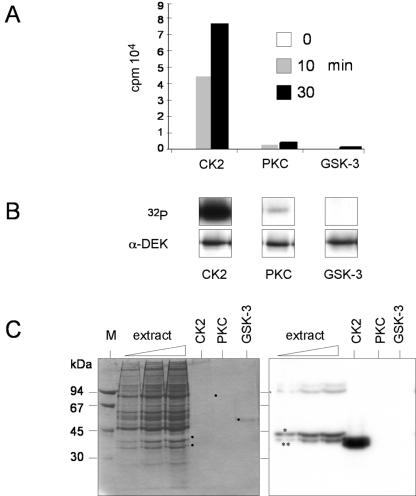
FIG. 2.
DEK is phosphorylated by CK2. (A) Filter binding assay. After dephosphorylation with λ-phosphatase, recombinant His-DEK (5 pmol) was incubated in the presence of [γ-32P]ATP and purified protein kinases (10 U). Reactions were stopped at the indicated time points, transferred to nitrocellulose filters, washed, and then counted. Incorporated radioactivity for the individual kinases is given in counts per minute (cpm, 104). (B) Products from the filter binding assay were analyzed by SDS-PAGE, autoradiography (32P), and Western blotting (α-DEK). (C) In-gel kinase assay. Increasing amounts of HeLa S-20 extract, together with purified CK2, PKC, and GSK-3, were subjected to electrophoresis in a 10% polyacrylamide gel that was polymerized in the presence of 40 μg of His-DEK per ml. The proteins were renatured, and the gel was incubated with [γ-32P]ATP. Shown are the Coomassie-stained gel (left) and the autoradiograph (right). Dots indicate the positions of purified kinases. The asterisk and double asterisk indicate the positions of the CK2 α and α′ subunits, respectively.