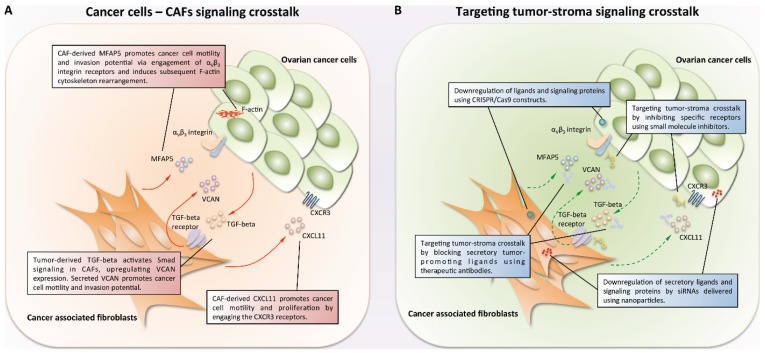
Figure 1.
Signaling crosstalk and its targeting in ovarian cancer. (A) Crosstalks between ovarian cancer cells and stromal CAFs promote tumor progression. Here, we illustrated three signaling crosstalk events between ovarian cancer cells and cancer-associated fibroblasts (CAFs). In the TGF-β-rich ovarian tumor microenvironment, Smad signaling was induced in CAFs through activation of TGF-β receptors. This stimulus increases the production of the CAF-derived secretory protein VCAN. VCAN, when acts on cancer cells, activates the NF-κB signaling, and subsequently promotes migration and invasion of cancer cells via the up-regulation of motility/invasion-related genes CD44, HMMR, and MMP9. On the other hand, CAF-derived MFAP5 binds to the αVβ3 integrin on the cancer cell and activates the calcium-dependent FAK/CREB/TNNC1 signaling pathways, which subsequently stimulate the reorganization of the F-actin cytoskeleton, thereby enhancing ovarian cancer cell motility. CAF-derived chemokine (C-X-C motif) ligand 11 (CXCL11) promotes cancer cell growth and migration via activation of the chemokine (C-X-C motif) receptor type 3 (CXCR3) on the cancer cell surface; and (B) multiple approaches can be used to target specific components within the cancer cell/CAF crosstalk signaling networks. Gene silencing can down-regulate the tumor- or stroma-derived secretory ligands that promote tumor progression by delivering specific gene-targeting siRNAs into tumor cells or CAFs using nanoparticles. Secretory factors from cancer cells and CAFs can be targeted using blocking antibodies. In addition, if specific receptors are involved in the crosstalk signaling, small molecule inhibitors can be used to inhibit the activation of these receptors. Lastly, gene editing using CRISPR/Cas9 technology can be developed into a therapeutic approach to down-regulate tumor- or stroma-derived secretory ligands and signaling molecules.