Abstract
Allogeneic hematopoietic cell transplantation (HCT) was originally developed to allow delivery of myeloablative doses of chemotherapy and radiotherapy. With better understanding of disease pathophysiology, the graft vs malignancy (GVM) effect of allogeneic hematopoietic transplantation and toxicities associated with myeloablative conditioning (MAC) regimens, the focus shifted to developing less toxic conditioning regimens to reduce treatment-related morbidity without compromising survival. Although HCT with MAC is preferred to reduced intensity conditioning (RIC) for most patients ≤ 60 years with AML/myelodysplastic syndrome and ALL, RIC and nonmyeloablative (NMA) regimens allow HCT for many otherwise ineligible patients. Reduced intensity preparative regimens have produced high rates of PFS for diagnoses, which are highly sensitive to GVM. Relapse of the malignancy is the major cause of treatment failure with RIC/NMA HCT. Incorporation of novel agents like bortezomib or lenalidomide, addition of cellular immunotherapy and use of targeted radiation therapies could further improve outcome. In this review, we discuss commonly used RIC/NMA regimens and promising novel regimens.
INTRODUCTION
Hematopoietic cell transplantation (HCT) is a potentially curative treatment option for a broad range of hematologic malignancies.1–3 Patients undergoing HCT receive a preparative regimen, also known as conditioning, with the goal of cytoreducing the malignancy and providing sufficient immunosuppression to prevent rejection of transplanted stem cells.4 Initial studies by Thomas et al.5 used supra-lethal doses of TBI; this approach could induce durable remission in a fraction of patients with advanced hematologic malignancies, but at the cost of substantial toxicity and nonrelapse mortality (NRM). Significant progress has been made in optimizing conditioning regimens to decrease the toxicity without compromising outcomes.
Traditional conditioning regimens or myeloablative conditioning (MAC) regimens comprised maximally tolerated doses of chemotherapy with or without radiation therapy with infusion of donor hematopoietic cells to provide hematologic and immune recovery. MAC regimens provide maximal direct anti-tumor activity, but at the expense of substantial nonhematopoietic toxicity. NRM with MAC regimens ranges from 10 to >50% depending on patient age, disease status, prior therapies, stem cell source and histocompatibility between the donor and the recipient.6–11 High NRM from MAC regimens precludes their use in elderly patients and in patients with major comorbidities. With the median age of patients with hematologic malignancies in the seventh decade, there is a need to provide a well-tolerated approach for HCT for older and medically infirm recipients. The decision to perform HCT depends on patient goals, comorbidities, donor availability and social support. Most centers choose reduced intensity conditioning (RIC)/nonmyeloablative (NMA) regimens for patients >60 years or those with major comorbidities. The HCT comorbidity index has been developed based on objective assessment of various organ functions. Geriatric assessment should also be performed pre-HCT including functional assessment, frailty and mental status. The number and severity of comorbidities predict outcomes post HCT.12,13
The therapeutic benefit of allogeneic HCT is largely derived from the immune graft vs malignancy (GVM) effect where donor immune cells eradicate residual malignant cells that may survive the preparative regimen.2,14–18 GVM is primarily mediated by donor T lymphocytes; however, it involves complex interaction between natural killer (NK) cells, dendritic cells and antibodies of donor origin.19,20 NK cell activity is particularly evident in T-cell-depleted, mismatched HLA transplants.21,22 Ab-mediated GVM is also described and may occur through C′-mediated cell death or by Ab-dependent cellular toxicity.20 Sensitivity of malignancy to GVM effect varies among different diseases, and depends on the diagnosis, tumor burden immune escape mechanisms and proliferation rate. This is summarized in Table 1. GVM seems most potent in CML, follicular lymphoma and CLL, while AML is intermediate and ALL is relatively insensitive to this effect.18,23,24 Pioneering work by Storb and McSweeney25 in canine models established that 200 cGy TBI was sufficiently immunosuppressive to allow engraftment of MHC-matched allogeneic HCT. This regimen is nonmyeloablative and autologous marrow recovery in recipients with graft failure.25 These preclinical studies led to the development of various RIC and NMA regimens to provide sufficient immunosuppression to achieve engraftment and allow GVM effects to eradicate the malignancy.
Table 1.
Disease sensitivity to GVM effects
| Highly sensitive | CML CLL Low-grade lymphoma Mantle cell lymphoma |
|
| |
| Intermediate sensitivity | AML Intermediate-grade lymphoma Hodgkin’s lymphoma Plasma cell myeloma |
| Relatively insensitive | ALL High-grade lymphoma |
Abbreviation: GVM = graft versus malignancy.
Champlin et al. suggested definitions of MAC, NMA and RIC preparative regimens. MAC regimens produce profound pancytopenia, which is usually irreversible and in most instances fatal, unless hematopoiesis is restored by HCT. In contrast, NMA regimens provide reversible myelosuppression, and autologous hematologic recovery would be expected to occur within 1 month without a HCT or if a graft is rejected. RIC regimens have intermediate intensity; they require a transplant for reliable hematopoietic recovery, but use a lower, less toxic dose of chemotherapy and radiation than traditional MAC regimens. Several forms of RIC regimens have been evaluated. The most common NMA regimens involve low-dose TBI, alone or in combination with fludarabine (Flu) or combinations of Flu and cyclophosphamide (Cy), with or without rituximab.26–31 RIC regimens have generally included an alkylating agent busulfan (Bu) or melphalan (Mel) in combination with a purine analog, typically Flu. More recently monoclonal antibodies, radioimmunotherapy or other agents have been incorporated into the conditioning regimens to better target and cytoreduce selected malignancies.
Specific definitions were adopted at the 1st International Workshop of Non-myeloablative Stem Cell Transplantation and adopted in 2006, at a workshop conducted by Center for International Blood and Marrow Transplant Research (CIBMTR).32 Bacigalupo et al.33 defined the intensity of various conditioning regimens in 2009. The intensities of most commonly used regimens are shown in Table 2. The relative myelosuppressive and immunosuppressive effects of various regimens are depicted in Figure 1.
Table 2.
Intensity of various conditioning regimens
| Myeloablative conditioning regimens | TBI ⩾ 5 Gy single dose or ⩾ 8 Gy fractionated Bu >8 mg/kg Cy200+ATG |
|
| |
| Nonmyeloablative conditioning regimens | TBI ≤ 2 Gy ± purine analog Flu+Cy ± rituximab +ATG Flu+AraC+Ida Cladribine+AraC Total lymphoid irradiation+ATG Flu+bendamustine+rituximab |
| Reduced intensity conditioning regimens | Flu Mel ± alemtuzumab or ATG Bu Flu |
Abbreviations: AraC = cytarabine; ATG = antithymocyte globulin; Bu = busulfan; Cy = cyclophosphamide; Flu = fludarabine; Gy = grays; Ida = idarubicin; Mel = melphalan.
Figure 1.
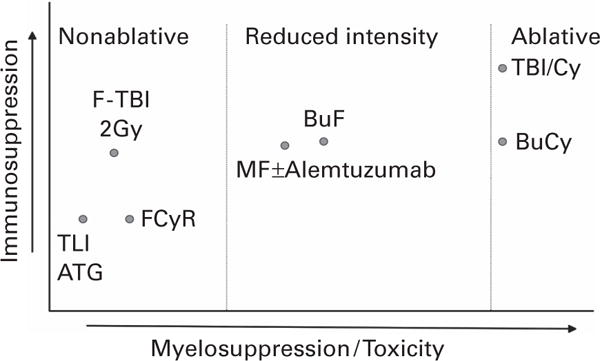
Relative myelosuppressive and immunosuppressive effects of various regimens. Bu = busulfan; Cy = cyclophosphamide; F = fludarabine; Gy = grays; Mel = melphalan; R = rituximab.
ADVANTAGES OF RIC AND NMA REGIMENS
RIC and NMA regimens allow HCT for patients considered ineligible for MAC because of advanced age or presence of comorbidities.34–38 RIC and NMA HCTs are generally better tolerated with lower rates of toxicity and NRM than occur with MAC HCTs.37,39–41
These regimens may also be associated with a reduced incidence and severity of acute GvHD.42 Several factors contribute to this observation. The clinical manifestations of acute GvHD reflect alloreactivity superimposed on the toxicity of the preparative regimen and subsequent cytokine release by damaged cells.43 Residual host T cells may produce a veto effect that inhibits the development of GvHD, which may be less severe in the setting of mixed chimerism. T-cell depletion with antithymocyte globulin (ATG) or alemtuzumab has been studied with RIC transplants using fludarabine and busulfan or melphalan. These agents decrease the incidence and severity of GvHD, but this benefit is offset by an increased risk of relapse and infections and overall survival has not improved.44
The same types of infectious complications occur as with MAC, but the severity appears to be reduced. Neutropenia is reduced or eliminated by NMA regimens.45 In addition, as the NMA preparative regimen does not immediately eliminate host-derived immunocompetent cells, these cells can contribute to host defense in the early post-transplant period.
A general recommendation is to utilize the least toxic regimen that can achieve the optimal therapeutic result. Thus, RIC and NMA regimens are indicated for diagnoses that are highly sensitive to GVM effects. In diagnoses where higher-dose intensity improves eradication of malignancy, RIC regimens should be reserved for elderly patients or those with comorbidities who could not tolerate an ablative regimen.24 The decision to use ablative or RIC should consider the patient’s age, performance status, frailty and comorbid conditions. There is no clearly defined age cutoff. Most centers recommend RIC or NMA regimens for patients over age 60 and most centers consider patients up to 75 years of age.
In this review, we summarize transplant outcomes with various RIC and NMA conditioning regimens in various hematologic malignancies and discuss novel conditioning regimens. Although most studies show comparable outcomes with RIC vs MAC for various diseases, they are generally retrospective studies with inherent selection bias. Bornhauser et al.46 published the first randomized study comparing outcomes of RIC vs MAC HCTs in patients with AML with the groups balanced for age, cytogenetic risk, induction therapy and donor type. Outcomes were similar in both groups with NRM, relapse, PFS and survival of 13 vs 18%, 28 vs 26%, 58 vs 56 and 61 vs 58%, respectively. Contrary to this study, BMT CTN 0901 was recently closed early in favor of MAC for AML/myelodysplastic syndrome (MDS) patients; detailed results have not yet been released. Results from a multicenter study by EBMT evaluating RIC vs MAC HCTs for MDS/secondary AML patients are eagerly awaited. Ideally, well-designed randomized trials for individual diseases comparing RIC vs MAC HCTs should be performed. Available single-arm studies and retrospective data must be interpreted with caution.
AML/MDS
HCT is a curative option for high-risk AML/MDS.47 AML is the most common indication for adult allogeneic transplants.48,49 Elderly age by itself and increased incidence of high-risk cytogenetics in elderly patients confer a poor prognosis. A study by CIBMTR and CALGB reported improvement in PFS in elderly patients receiving RIC hematopoietic transplantation compared with those receiving chemotherapy alone.50 Age did not independently affect outcomes of elderly patients with AML and MDS undergoing HCT with RIC regimens.51 Several studies have shown that RIC transplantation is a feasible option for otherwise transplant ineligible patients and outcomes primarily depend on the disease status at the time of HCT. RIC improved overall and disease-free survival compared with patients treated with conventional chemotherapy regimens as summarized in Table 3.52–67 The overall survival ranged from 42 to 79%, PFS from 37 to 76% and NRM from 4 to 33%. Patients receiving RIC transplants had similar outcomes compared with patients who had transplant with MAC regimens for AML and MDS are summarized in Table 4.68–71
Table 3.
Summary of HCT studies with RIC/NMA regimens
| Study | Disease | Regimen | aGvHD 2–4 (%) | cGvHD (%) | TRM (%) | Relapse (%) | PFS (%) | OS (%) |
|---|---|---|---|---|---|---|---|---|
| Van Besien52 | AML/MDS | Flu Mel Ale | 33 | 18 | 33 | 27 | 38 | 48 (1 yr) |
| Wong53 | AML/MDS/CML | Flu Mel | 41 | 63 | 55 | NR | 37 | 44 (27 mo) |
| Taussig54 | AML/MDS | Flu Cy/Flu Mel | 12 | 66 | 0(100 d) | NR | 56% | 69 (26 mo) |
| Alatrash55 | AML/MDS | Flu Bu | 41 | 43 | 26(3 yr) | NR | 44 | 46 (2 yr) |
| Nakamura56 | AML/MDS | Flu Mel | 63 | 62 | 35 | 16 | 51 | 53 (2 yr) |
| Blaise57 | AML | Flu Bu ATG | 24 | 70 | 9 | 18 | 76 | 79 (18 mo) |
| Grigg58 | AML | Flu Cy | 30 | 67 | 15 | 37 | 56 | 68 (2 yr) |
| Valcarcel59 | AML | Flu Bu | 35 | 53 | 20 | 44 | 39 | 42 (4 yr) |
| Kohrt60 | AML | TLI ATG | 10 | 28 | 4(1 yr) | NR | 48 | 50 (3 yr) |
| Martino77 | ALL | Flu Mel/Flu Cy | 63 | 72 | 23 | 49 | NR | 31 (2 yr) |
| Hamaki78 | ALL | Flu Bu/Flu Mel | 45 | 64 | 30 | 51 | 30 | 40 (1 yr) |
| Mohty79 | ALL | Flu TBI/Flu Mel/Flu Cy | NR | NR | 28 | 51 | 21 | 31 (2.8 yr) |
| Bachanova80 | ALL | Flu Cy TBI | 55 | 45 | 27 | 36 | NR | 50 (3 yr) |
| Ram81 | ALL | Flu TBI | 53 | 44 | 28 | 40 | NR | 34 (3 yr) |
| Stein82 | ALL | Flu Mel | 75 | 86 | 21 | 21 | 61 | 61 (2 yr) |
| Cho83 | ALL | Flu Mel | 43 | 65 | 18 | 20 | 63 | 64 (3 yr) |
| Sorror103 | CLL | 2 Gy TBI | 55–66 | 49–53 | 23 | 38 | 39 | 50 (5 yr) |
| Khouri104 | CLL | Flu Cy/Flu Mel R | 37 | 56 | 17 | 39 | 36 | 51 (5 yr) |
| Dreger105 | CLL | Flu Cy based | 45 | 73 | 23 | 40 | 42 | 65 (4 yr) |
| Michallet106 | CLL | Flu TBI | 44 | 29 | NR | 22 | 46 | 55 (3 yr) |
| Giralt109 | Myeloma | Flu Mel | 46 | 28 | 40 | NR | 19 | 30 (2 yr) |
| Mohty110 | Myeloma | Flu Bu ATG | 36 | 41 | 17 | 51 | 41 | 62 (2 yr) |
| Gerull111 | Myeloma | Flu TBI 2 Gy | 37 | 70 (18 mo) | 17 | NR | 29 | 41 (18 mo) |
| Kroger112 | Myeloma | Flu Mel ATG | 38 | 37 | 26 | 26/86 | 54 | 73 (2 yr) |
| Khouri115 | FL | FCR | 11 | 60 | 15 | 2 | 83 | 85 (5 yr) |
| Khouri116 | FL | 90YFC | 13 | 39 | 8 | NR | 85 | 88 (3 yr) |
| Pinana117 | FL | Flu Mel | 53 | 78 | 41 | 8 | 57 | 54 (4 yr) |
| Vigouroux118 | FL | Flu based | 34 | 43 | 32 | 9.6 | 66 | 66 (3 yr) |
| Cook119 | MCL | Flu Mel+/− Ale/Flu Bu +/− Ale | 39 | 61 | 21 | 65 | 14 | 37 (5 yr) |
| Rezvani120 | DLBCL | Flu TBI | 53 | 47 | 25 | 41 | 35 | 45 (3 yr) |
| Thomson121 | DLBCL | Flu Mel Ale | 17 | 22 | 34 | 33 | 48 | 47 (4 yr) |
| Sirvent122 | DLBCL | Flu Bu/Flu Cy/Flu Mel/Flu TBI | 39 | 41 | 23 | 41 | 44 | 49 (2 yr) |
| Alvarez131 | HL | Flu Mel | 45 | 45 | 25 | NR | 32 | 48 (2 yr) |
| Sureda132 | HL | Flu Mel +/− ATG | 48 | 47 | 15 | 37 | 24 | 43 (4 yr) |
| Anderlini137 | HL | Gem-Flu Mel | 19 | 39 | 15 | NR | 55 | 78 (18 mo) |
Abbreviations: aGvHD = acute GvHD; Ale = alemtuzumab; ATG = antithymocyte globulin; Bu = busulfan; cGvHD = chronic GvHD; Cy = cyclophosphamide; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; Flu = fludarabine; Gem = gemcitabine; HCT = hematopoietic cell transplantation; HL = Hodgkin’s lymphoma; MAC = myeloablative conditioning; MDS = myelodysplastic syndrome; MCL = mantle cell lymphoma; Mel = melphalan; mo = months; NMA = nonmyeloablative; NR = not reported; OS = overall survival; RIC = reduced intensity conditioning; TRM = treatment-related mortality.
Table 4.
Summary of studies comparing RIC with MAC regimens
| Study | Disease | RIC vs MAC aGvHD 2–4 (%) | RIC vs MAC cGvHD (%) | RIC vs MAC NRM (%) | RIC vs MAC Relapse (%) | RIC vs MAC PFS (%) | RIC vs MAC OS (%) |
|---|---|---|---|---|---|---|---|
| Olle Ringden68 | AML | 31 vs 36 | 31 vs 38 | HR 0.85 | HR 1.46 | HR 0.88 | NR |
| AML | 29 vs 29 | 36 vs 46 | HR 0.64 | HR 1.34 | HR 1.04 | NR | |
| Shimoni A69 | AML | 8 vs 22 | 31 vs 56 | 22 vs 8 | 14 vs 9 | 45 vs 49 | 50 vs 49 (2 yr) P = NS |
| Flynn CM70 | AML | 22 vs 17 | 13 vs 18 | 34 vs 33 | RR 2.2 | 31 vs 30 | 33 vs 35 (2 yr) P>0.1 |
| Aoudjhane M71 | AML | 22 vs 31 | 48 vs 56 | 32 vs 18 | 41 vs 24 | 40 vs 47 | 44 vs 46 (2 yr) P = NS |
| Martino63 | AML/MDS | 43 vs 58 | 45 vs 52 | 32 vs 22 | 26 vs 40 | 33 vs 39 | 41 vs 45 (3 yr) P = 0.8 |
| Luger64 | AML/MDS | 41 vs 45 | 40 vs 35 | RR 0.9 | 39 vs 32 | NR | 33 vs 34 (5 yr) P = NS |
| Scott65 | AML/MDS | 54 vs 78 | 55 vs 64 | 41 vs 34 | 31 vs 23 | 27 vs 44 | 28 vs 48 (3 yr) P = 0.56 |
| Alyea27 | AML/MDS | 26 vs 27 | 33 vs 18 | 15 vs 32 | 61 vs 38 | 20 vs 31 | 34 vs 28 (2 yr) P = 0.056 |
| Eom85 | ALL | 51 vs 52 | 61 vs 50 | 21 vs 24 | 34 vs 26 | 51 vs 55 | HR 0.98 (5 yr) P = 0.30 |
| Marks86 | ALL | 39 vs 46 | 34 vs 42 | 32 vs 33 | 35 vs 26 | 32 vs 41 | 38 vs 43 (3 yr) P = 0.39 |
| Mohty87 | ALL | Similar | Similar | 21 vs 29 | 47 vs 31 | 32 vs 38 | 45 vs 48 (2 yr) P = 0.23 |
| Bachanova88 | ALL | 46 vs 41 | 63 vs 45 | 13 vs 36 | 49 vs 28 | 26 vs28 | 39 vs 35 (3 yr) P = 0.62 |
| Crawley113 | Myeloma | 35 vs 46 | Similar | 24 vs 37 | 19 vs 34 | 38 vs 51 (2 yr) P = NS | |
| Hari123 | FL | 44 vs 36 | 62 vs 46 | 23 vs 23 | RR 2.97 | 55 vs 67 | 62 vs 71 (3 yr) P = 0.15 |
| Hamadani128 | MCL | 37 vs 36 | 43 vs 35 | 43 vs 47 | 32 vs 33 | 25 vs 20 | 30 vs 25 (3 yr) P = 0.45 |
| Peres107 | CLL | 39 vs 55 | 38 vs 48 | 14 vs 27 | 15 vs 14 | NR | 63 vs 18 (5 yr) P = 0.006 |
| Brown108 | CLL | 30 vs 50 | NR | 46 vs 9.5 | 7 vs 26 | NR | 63 vs 49 (5 yr) P = 0.003 |
| Sureda133 | HL | 44 vs 53 | 38 vs 40 | 23 vs 46 | 57 vs 30 | 18 vs 20 | 28 vs 22 (5 yr) P = 0.04 |
| Bacher125 | DLBCL | 43 vs 43 | 42 vs 37 | 47 vs 56 | 38 vs 26 | 15 vs 18 | 20 vs 18 (5 yr) P = NR |
Abbreviations: aGvHD = acute GvHD; cGvHD = chronic GvHD; DLBCL = diffuse large B-cell lymphoma; FL = follicular lymphoma; HL = Hodgkin’s lymphoma; HR = hazard ratio; MAC = myeloablative conditioning; MCL = mantle cell lymphoma; MDS = myelodysplastic syndrome; mo = months; NR = not reported; NRM = nonrelapse mortality; OS = overall survival; RIC = reduced intensity conditioning; yr = years.
In an attempt to further reduce NRM, NMA transplants have been evaluated. NMA regimen transplants can achieve long-term remission in elderly patients with poor performance status, but the results appear inferior when compared with RIC regimen transplants for patients who are not in remission.72,73 All these studies had heterogeneous groups of patients, inherent bias of retrospective studies and comorbidities were not taken into account during analyses and they must be interpreted with caution. Prospective multicenter studies randomizing patients to RIC vs MAC regimen transplantation are currently ongoing in Europe.
ALL
Patients with ALL achieve high rates of CR with modern chemotherapy, but long-term disease remission is seen only in a one-third of patients. Consolidation treatment with HCT has improved outcomes.74,75 ALL is a relatively insensitive disease to GVM effect;24 however, patients who develop GvHD do have a reduced risk of relapse. A large prospective study by UK Medical Research Council (MRC)/Eastern Cooperative Oncology Group (ECOG), MRC UKALL XII/ECOG E2993 showed improvement in a 5-year survival for standard risk ALL patients receiving HCT 53 vs 45% for those with no donor and received conventional chemotherapy. However, in patients with high-risk ALL donor vs no donor 5-year survival was not significantly different (41 vs 35%, P = 0.2) due to high NRM of 36% at 2 years.76 RIC regimen transplants are a reasonable option for patients who are not candidates for MAC regimen transplants as shown in Table 3.77–84 Flu-Mel conditioning regimen provided survival of >60% with NRM and relapse of around 20% each.
Outcomes of RIC transplants were compared with patients who had MAC regimens. Similar results occurred in patients with Philadelphia-negative ALL, while patients with Philadelphia-positive disease had a higher incidence of relapse with RIC. This is summarized in Table 4.85–88 Incorporation of tyrosine kinase inhibitor (TKI) treatment may improve outcomes of patients with Philadelphia-positive ALL undergoing RIC. Bachanova et al.88 reported superior survival 55 vs 33% with RIC compared with MAC with use of pre-HCT TKI in patients who have negative minimal residual disease.
CML
CML was the most common indication for HCT in the pre-TKI era and is the disease where there is best evidence of a potent graft vs tumor effect17. RIC regimen transplants are effective treatment options for CML patients refractory to TKI therapy.89–94 Outcomes of CML with RIC HCT depend on stage of disease; best results are reported in patients in chronic phase where outcomes are similar compared with MAC transplants.94 However, RIC transplants may not be adequate for advanced stage disease.92,93 TKI treatment and donor lymphocyte infusions have been effective for patients with residual disease after RIC transplants.
CLL
CLL is also susceptible to the graft vs tumor effect.17,95–102 RIC and NMA regimen HCTs have shown encouraging results with survival ranging between 50 and 65% as shown in Tables 3 and 4.103–108 Khouri et al.104 demonstrated Flu-Cy conditioning and immunomodulation with rituximab, withdrawal of immunosuppression and donor lymphocyte infusion (DLI) in patients with relapsed refractory CLL achieved survival of 51% and in certain HLA genotype patients the PFS was 68% at 5 years. This regimen was well tolerated with NRM of 17%.
PLASMA CELL MYELOMA
Allogeneic HCT has been extensively evaluated for treatment of plasma cell myeloma. These patients tend to be relatively frail and MAC HCTs have been associated with a high rate of NRM. RIC HCT has been the preferred approach for heavily pretreated patients eligible for allogeneic transplants in salvage settings post autologous HCT and summarized in Tables 3 and 4.109–112 Outcomes of RIC and MAC are similar. There is a higher rate of relapse with RIC transplants, which offsets the benefit of decreased NRM.113 Preliminary studies are examining incorporation of bortezomib and lenalidomide into conditioning regimens; prospective trials are needed.114
LYMPHOMA
Lymphomas have variable degrees of sensitivity to GVM effects, with low-grade lymphomas having highest sensitivity and large-cell lymphoma and Hodgkin’s disease being relatively insensitive. There are data to support use of RIC transplant in patients with various non Hodgkin’s lymphoma (NHL) and like most other conditions, outcomes of RIC transplants are similar to MAC transplants as summarized in Tables 3 and 4.115–125 More recently, targeted therapies and radioimmunotherapy have been incorporated into RIC regimens to decrease the tumor burden of patients undergoing transplants, thereby decreasing risk of relapse and not adversely affecting NRM.
Relapsed refractory follicular lymphoma can be cured with NMA HCT. Khouri et al.115 reported data of 47 patients treated at MD Anderson cancer center (MDACC) with Flu, Cy and rituximab (FCR) conditioning. At a median follow-up of 11 years survival was 78% and PFS was 72%. NRM was low at only 15 %. Two other studies, by Thompson et al.126,127 and CALGB with RIC HCT using Flu-Mel conditioning, with or without Alemtuzumab showed survival of 76–81% and PFS of 75% with over 3-year median follow-up.
NMA HCT is feasible and effective for patients with relapsed refractory mantle cell lymphoma. With FCR conditioning, Khouri et al.115 reported PFS of 43% and survival of 53% at 6 years. Results reported by CIBMTR and Cook et al.119,128 showed less encouraging outcomes with survival ranging between 14 and 25% at 3-year follow-up, which could be related to differences in number of patients with chemosensitive disease and relapsed disease after autologous stem cell transplantation.
More recently, the group from MD Anderson Cancer Center presented phase II data with NMA conditioning HCT using bendamustine, Flu and rituximab in CLL and NHL Survival and PFS were 89 and 80% with NRM of 9% with a median follow-up of 1 year.129 This regimen is well tolerated with a low incidence of cytopenias. It can be used as a conditioning for outpatient transplants.
In the last decade radioimmunotherapy has emerged as a promising treatment option for hematological diseases especially chemoresistant NHL. Radiolabeled monoclonal antibodies allow for targeted therapy with low-toxicity rates and high-response rates when used as single agents. Addition of 90Y-ibritumomab tiuxetan to Flu-TBI regimen was evaluated in heavily pretreated aggressive NHL patients by Gopal et al.130 Overall Survival, PFS and NRM were 54.1, 31.1 and 15.9%, respectively, at 30 months. The NRM at day 100 was only 2.5% and this strategy could induce early responses in patients who otherwise had refractory disease. This strategy would allow adequate time for harnessing benefit from GVM effect.
Khouri et al. reported data with incorporation of 90Y-ibritumomab tiuxetan to fludarabine and cyclophosphamide for chemorefractory follicular lymphoma. Results were encouraging with the 3-year PFS rates of 80 and 87% with chemorefractory or chemosensitive disease. This strategy could be attempted in other CD20-positive malignancies refractory to salvage chemotherapy regimen.
Promising new therapies like epratuzumab an anti-CD22 mAb conjugated with 90Y showed excellent response in refractory follicular lymphoma patients. Inotuzumab ozogamicin, an antibody drug conjugate with anti-CD22 mAb in patients with ALL, produced an overall response rate of >50% in treatment-refractory patients. Addition of these agents to RIC is being evaluated in ongoing clinical trials.
HODGKIN’S LYMPHOMA
Despite a relatively low sensitivity to GVM, RIC transplants in Hodgkin’s lymphoma have produced durable remissions in 30–40%, as summarized in the Tables 3 and 4.131–134 Most patients had relapsed after autologous hematopoietic transplants, before being referred for allogeneic transplantation. Most long-term responders had chemosensitive low bulk disease. The Flu-Mel RIC regimen has been commonly used; this resulted in NRM <15% in matched related and unrelated transplants.135 Relapse is the main cause of treatment failure in patients who have HCT, and risk of relapse depends on chemosensitivity of disease, poor performance status, age >45, relapse <6 months after autologous transplantation and transplantation before 2002.136
With the availability of brentuximab vedotin, salvage therapy was more effective in increasing CR rates before allogeneic transplantation. The addition of gemcitabine to fludarabine-melphalan conditioning showed encouraging results with PFS and survival at 2 years of 55 and 78%, respectively. Incidence of NRM was acceptable at 15%.137
SPECIFIC PREPARATIVE REGIMENS
Fludarabine melphalan (Flu-Mel)
This regimen was pioneered by Giralt et al.,138 based on the idea that melphalan has activity in variety of hematological malignancies and is well tolerated in elderly patients even at high doses. Purine analogs exert a synergistic effect by inhibiting mechanisms of DNA repair from alkylating agent-induced DNA damage, and, in combination with the alkylating agent, have adequate immunosuppressive effect to allow engraftment. The median age of patients was 52 years (range, 22–70 years). AML patients in first remission or CML patients in chronic phase had 57% disease-free survival at 1 year. Grade III–IV aGvHD was seen in 19% of patients who had related donor transplants vs 39% in patients who had unrelated donor transplants. NRM rate was 37.4% at day 100. This regimen has activity in both lymphoid and myeloid malignancies.139 Several other investigators have reported decreased NRM with Flu-Mel conditioning in patients, otherwise not eligible for HCT for various hematological malignancies as shown in disease-specific tables.
GvHD and treatment of GvHD causing mortality from various infections are a concern with RIC Flu-Mel conditioning. Alemtuzumab, a monoclonal antibody against CD52, has been combined with Flu-Mel conditioning to decrease incidence and severity of GvHD; however, several studies showed increased relapse rates needing donor lymphocyte infusion and also increased risk of infections.140–142 Van Beisen et al. compared outcomes of AML/MDS patients treated with Flu-Mel regimen and Flu-Mel +alemtuzumab regimen treated at two different sites. NRM, relapse, survival and disease-free survival were similar in both groups. Grade II–IV acute GvHD and chronic GvHD was significantly lower in patients who received Flu-Mel+Alemtuzumab.66 In a CIBMTR analysis, patients who received alemtuzumab for in vivo T-cell depletion effectively reduced GvHD compared with T-cell replete grafts, with grades 2–4 and 3–4 acute GvHD 40 vs 19%, P = 0.001, and 22 vs 11%, P = 0.001; however, this did not translate into better overall survival because of increased recurrence rates.143 In another study, patients with AML treated with RIC and alemtuzumab had worse overall survival compared with patients treated with T-cell-replete grafts despite lower rates of GvHD because of loss of GVM effect.144 Recently Gartner et al.145 reported quicker NK cell recovery with lower dose of alemtuzumab with sufficient GvHD prophylaxis, which might improve survival by decreasing risk of infections and relapse. For indolent diseases like follicular lymphoma where NRM is higher than risk of disease progression, use of donor lymphocyte infusion for patients with relapse has helped in decreasing risk of relapse with acceptable NRM of 15% at 4 years and PFS of 76%.126
Another in vivo T-cell depletion strategy is the use of ATG, which has been extensively studied in unrelated donor transplants with RIC regimens. In the CIBMTR study cited above, rates of grades 2–4 and grades 3–4 acute GvHD were similar with T-cell replete and ATG-containing regimens: 40 vs 38% and 22 vs 21%, respectively, and the overall survival rate was lowest in the patients receiving ATG compared with alemtuzumab and T-cell-replete grafts, 38 vs 50% and 46%. There was no advantage seen in the NRM rates with ATG-containing RIC regimens. Also patients who received ATG had a higher incidence of Epstein Barr virus post-transplant lymphoproliferative disorder unlike alemtuzumab, which effectively depletes B cells. Despite improvement of supportive care and decrease in infections with antimicrobial therapies we recommend cautious use of T-cell depletion strategies based on the disease status, chemosensitivity and pre-transplant therapies, given the above data.
Fludarabine busulfan
Low-dose Bu with Flu was first developed as a conditioning regimen, with the Bu to achieve well-tolerated cytotoxicity against myeloid malignancies in combination with Flu and ATG as immunosuppressive agents to prevent rejection of infused stem cells.97 Since then variations of this regimen have been studied extensively by several transplant groups for both myeloid and lymphoid malignancies and are summarized in disease-specific tables. This regimen was studied by Brenner et al.146 in patients 70 years or older patients and the cumulative incidence of grades II to IV acute GvHD was 13% and grades III to IV was 9.3%. At 2 years the cumulative incidence of chronic GvHD was 36%, survival and PFS were 39%, with relapse rate of 56%. NRM was 3.7% at day 100 and 5.6% at one year. Within the RIC regimen two different doses of Bu, 3.2 and 6.4 mg/kg, IV were studied with Flu by Chen et al.147 in patients with MDS/AML. The cumulative incidences of acute GvHD and chronic GvHD were similar. Two-year NRM rates were 8.9 vs 9.8%, respectively; PFS 40.6 vs 39.3% and survival 47.4 vs 48.8%, respectively, predicting dose of 3.2 mg/kg might be sufficient in most cases.
The Flu-Bu conditioning regimen was often used in combination with ATG with the goal of improving NRM and GvHD rates, A large French study with addition showed significant improvement in GvHD rates with addition of rabbit ATG 5mg/kg to Flu-Bu regimen for AML patients. At 4 years of follow-up grade III–IV acute GvHD was 9%, and extensive chronic GvHD occurred in 22%. The survival at 4 years was 54% with relapse rate of 36% and NRM of 22%.148 These results are promising and further strategies to decrease relapse rates could improve outcomes further.
Low-dose TBI+/− fludarabine
TBI alone with or without Flu as a NMA conditioning regimen was pioneered by Storb and coworkers, primarily to transplant patients who are >60 years of age. They reported 372 patients between the ages 60 and 75; cumulative incidences of NRM and relapse at 5 years were 27 and 41%, respectively, with 5-year survival of 35% and PFS of 32%. Higher age was not associated with the higher incidence of acute GvHD or chronic GvHD. Analysis based on age did not significantly affect the outcomes and it was the disease risk and comorbidity index, which were the determinants.149 Outcomes of patients who were treated with TBI alone vs TBI along with Flu were compared in a randomized phase III study. Outcomes were superior with addition of Flu to TBI regimen with 3-year survival 65 vs 54% in favor of Flu-TBI and they had lower relapse rate 40 vs 55%. Addition of Flu contributed to better T-cell and NK-cell chimerism at day 28 and less incidence of graft failure.150 Transplant with Flu TBI conditioning is a reasonable option in elderly patients who would otherwise be not eligible for transplantation.
Total lymphoid irradiation/ATG
Investigators from Stanford university developed a novel RIC regimen with total lymphoid irradiation and ATG, which altered host immunity profile to favor regulatory NK T cells. A total of 111 (67 lymphoid/34 myeloid) patients were treated with total lymphoid irradiation/ATG and received G-CSF-mobilized grafts. Over a third of the patients in this study were >60 years of age, with half of the lymphoid malignancy patients having high risk of relapse after failing autologous transplantation. Fifty-one of these patients had mismatched donors. Cumulative incidence of grade II–IV GvHD was 2 and 10% for related and unrelated donors, and 1-year NRM was significantly improved at 4%. Probability of 3-year survival and PFS was 60 and 40%, respectively.60
Treosulfan-based regimens
Treosulfan is a prodrug of a bifunctional alkylating cytotoxic agent that is approved for the treatment of ovarian cancer in Europe. Treosulfan-based reduced toxicity regimens were well studied in the myeloablative setting, Michallet et al.151 studied treosulfan fludarabine ATG RIC regimen in a phase II trial. This study included patients with acute and chronic leukemias. The regimen was well tolerated with NRM of 23% at 2 years and overall survival of 52% at 3 years. Cumulative incidence of relapse was 25%, and of acute and chronic GvHD was around 30%.
Clofarabine-based regimens
Clofarabine is a second generation, purine analog with activity in relapsed AML with favorable toxicity profile. It has been studied as an alternative to fludarabine for RIC. Debulking relapsed/refractory AML/MDS patients with clofarabine/cytarabine followed by RIC HCT with Cy TBI led to 2-year PFS and overall survival of 52 and 56%, respectively.152 Small prospective study CLORIC trial with clofarabine busulfan RIC in high-risk AML/MDS, ALL and biphenotypic leukemia patients with median age of 59 years showed excellent 1-year PFS and overall survival of 63 and 57%, respectively.153 Several other studies confirmed feasibility and efficacy of clofarabine-based conditioning.154,155
FUTURE DIRECTIONS
Better understanding of molecular pathways led to identification of treatment targets in various hematological malignancies and has improved response rates in frontline and relapse settings. Outcomes of RIC/NMA HCTs could be further improved by incorporation of novel agents to the disease-specific conditioning regimens without significant additional toxicities.
Targeted radioimmunotherapy
Pagel et al.156 pioneered use of 131I-labeled anti-CD45 antibody in combination with a NMA regimen of fludarabine and low-dose TBI to deliver targeted radiation to the bone marrow, spleen and lymph nodes in patients with AML or MDS who had >5% blasts before transplant. The authors demonstrated that the maximal tolerated dose (MTD) of radiation to the liver was 24 Gy by radio immunotherapy, which would otherwise be a supralethal dose when administered by conventional methods. A third of these patients had infusional reactions; however, they resolved at the end of infusion. Results of this study were encouraging with 100% T and myeloid donor engraftment at 1 month with NRM of 12% at 100 days. The relapse rate was 40% and overall survival of 41% at 1 year. Similarly Ringhoffer et al.157 demonstrated that 188Re or 90Y-labeled anti-CD66 antibody delivered radiation dose of 21.9 (+/− 8.4) Gy to the marrow with a significantly higher dose using 90Y conjugate and had encouraging results when used as a part of conditioning therapy. However, these agents are β-particle-emitting isotopes, which create a field radiation effect and target negative marrow tumor cells. These agents could also cause non-specific toxicity to normal cells. α-Particles have higher linear energy transfer and shorter range with less non-specific toxicity.158 α-Emitters 213Bi and 225Ac in combination with targeting vehicle-humanized anti-CD33 mAb Lintuzumab are being evaluated in phase I/II studies alone and in combination in AML.159–161 Preliminary results show encouraging reduction in bone marrow blast percentage. This strategy with 213Bi-labeled Abs, against CD45 and T-cell receptor αβ before transplantation, is being tested in canine models to minimize the toxicity of NMA regimens.162,163
Pretargeted radioimmunotherapy
Pretargeted radioimmunotherapy is an interesting strategy, which could further reduce the systemic toxicities to normal organs with encouraging results in pre-clinical setting. Targeting molecule anti-CD45 mAb conjugated with streptavidin is infused to reach the target cells, and circulating Ab is removed by ‘Clearing agent’. This followed by infusion of 213Bi- or 90Y-DOTA-biotin led to leukemia-free survival in mouse models up to 100 days.164 Other interesting strategies include incorporation of bispecific Ab-hapten in NHL patients and single-walled carbon nanotubes (SWNTs) with complementary morpholino oligonucleotide (MORF) sequences.165,166 SWNT-MORF bind to cancer cells with significantly higher affinity than monoclonal Abs as they have been pretargeted with antibodies modified with oligonucleotide strands complementary to those on the nanotubes. Inclusion of these agents with conditioning could further improve outcomes especially in patients with residual disease at the time of HCT.
Helical tomotherapy
Helical tomotherapy involves delivery of radiation therapy from a rotating beam source and has the advantage of safe dose escalation to bone marrow, spleen and lymphoid tissues with significantly lower radiation damage to normal tissues. Chargari et al.167 demonstrated the feasibility of HT as a preparative regimen for HCT to debulk patients with lymphoma.
CONCLUSIONS
RIC and NMA regimens provide the opportunity for hematopoietic transplants for elderly patients and in those with comorbidities who cannot tolerate MAC. The relative role of RIC vs MAC needs to be determined for each diagnosis, ideally by randomized clinical trials. Preliminary data suggest that RIC regimens are highly effective for indolent lymphoid malignancies and may be preferred for these diagnoses. In contrast, the intensity of the preparative regimen appears important in myeloid malignancies, particularly for those with active disease. The major causes of treatment failure with RIC transplants are relapse and GvHD. Novel approaches to selectively cytoreduce the malignancy or enhance GVM immune effects are needed to improve transplant outcome. Preclinical and early clinical data with targeted radiation therapy approaches are promising. Improved approaches to control GvHD and infections are also needed to improve overall survival. Controlled clinical trials are required to define optimal therapeutic strategies.
Footnotes
CONFLICT OF INTEREST
Dr Champlin has had research grant funding from Otsuka, Sanofi and Celgene Corporations.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Gale RP, Champlin RE. How does bone-marrow transplantation cure leukaemia? Lancet. 1984;2:28–30. doi: 10.1016/s0140-6736(84)92009-9. [DOI] [PubMed] [Google Scholar]
- 3.Thomas ED. Karnofsky Memorial Lecture. Marrow transplantation for malignant diseases. J Clin Oncol. 1983;1:517–531. doi: 10.1200/JCO.1983.1.9.517. [DOI] [PubMed] [Google Scholar]
- 4.Vriesendorp HM. Aims of conditioning. Exp Hematol. 2003;31:844–854. doi: 10.1016/s0301-472x(03)00229-7. [DOI] [PubMed] [Google Scholar]
- 5.Thomas ED, Lochte HL, Jr, Cannon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clift RA, Buckner CD, Appelbaum FR, Bearman SI, Petersen FB, Fisher LD, et al. Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens. Blood. 1990;76:1867–1871. [PubMed] [Google Scholar]
- 7.Pino Y, Torres JL, Bross DS, Lam WC, Wharam MD, Santos GW, et al. Risk factors in interstitial pneumonitis following allogenic bone marrow transplantation. Int J Radiat Oncol Biol Phys. 1982;8:1301–1307. doi: 10.1016/0360-3016(82)90579-x. [DOI] [PubMed] [Google Scholar]
- 8.Hill-Kayser CE, Plastaras JP, Tochner Z, Glatstein E. TBI during BM and SCT: review of the past, discussion of the present and consideration of future directions. Bone Marrow Transplant. 2011;46:475–484. doi: 10.1038/bmt.2010.280. [DOI] [PubMed] [Google Scholar]
- 9.Bensinger WI, Clift R, Martin P, Appelbaum FR, Demirer T, Gooley T, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996;88:2794–2800. [PubMed] [Google Scholar]
- 10.Hassan M. The role of busulfan in bone marrow transplantation. Med Oncol. 1999;16:166–176. doi: 10.1007/BF02906128. [DOI] [PubMed] [Google Scholar]
- 11.Ringden O, Remberger M, Ruutu T, Nikoskelainen J, Volin L, Vindelov L, et al. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic Bone Marrow Transplantation Group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 12.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muffly LS, Kocherginsky M, Stock W, Chu Q, Bishop MR, Godley LA, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99:1373–1379. doi: 10.3324/haematol.2014.103655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apperley JF, Mauro FR, Goldman JM, Gregory W, Arthur CK, Hows J, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988;69:239–245. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]
- 15.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 16.Odom LF, August CS, Githens JH, Humbert JR, Morse H, Peakman D, et al. Remission of relapsed leukaemia during a graft-versus-host reaction. A “graft-versus-leukaemia reaction” in man? Lancet. 1978;2:537–540. doi: 10.1016/s0140-6736(78)92879-9. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–1728. [PubMed] [Google Scholar]
- 18.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 19.Porter DL. Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. Hematology Am Soc Hematol Educ Program. 2011;2011:292–298. doi: 10.1182/asheducation-2011.1.292. [DOI] [PubMed] [Google Scholar]
- 20.Miller JS, Warren EH, van den Brink MR, Ritz J, Shlomchik WD, Murphy WJ, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT: Graft-versus-Tumor/Leukemia Reaction. Biol Blood Marrow Transplant. 2010;16:565–586. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 22.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins RH, Jr, Goldstein S, Giralt S, Levine J, Porter D, Drobyski W, et al. Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant. 2000;26:511–516. doi: 10.1038/sj.bmt.1702555. [DOI] [PubMed] [Google Scholar]
- 24.Kahl C, Storer BE, Sandmaier BM, Mielcarek M, Maris MB, Blume KG, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. doi: 10.1182/blood-2007-03-078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McSweeney PA, Storb R. Mixed chimerism: preclinical studies and clinical applications. Biol Blood Marrow Transplant. 1999;5:192–203. doi: 10.1053/bbmt.1999.v5.pm10465099. [DOI] [PubMed] [Google Scholar]
- 26.McClune BL, Weisdorf DJ. Reduced-intensity conditioning allogeneic stem cell transplantation for older adults: is it the standard of care? Curr Opin Hematol. 2010;17:133–138. doi: 10.1097/MOH.0b013e3283366ba4. [DOI] [PubMed] [Google Scholar]
- 27.Alyea EP, Kim HT, Ho V, Cutler C, Gribben J, DeAngelo DJ, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 28.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104:1550–1558. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 29.Belkacemi Y, Labopin M, Hennequin C, Hoffstetter S, Mungai R, Wygoda M, et al. Reduced-intensity conditioning regimen using low-dose total body irradiation before allogeneic transplant for hematologic malignancies: Experience from the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys. 2007;67:544–551. doi: 10.1016/j.ijrobp.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 30.Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM, et al. Low-dose total body irradiation (TBI) and fludarabine followed by hematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with hematological diseases. Blood. 2003;101:1620–1629. doi: 10.1182/blood-2002-05-1340. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Kanda Y, Kami M, Kato K, Shoji N, Kanai S, et al. Therapeutic potential of a reduced-intensity preparative regimen for allogeneic transplantation with cladribine, busulfan, and antithymocyte globulin against advanced/refractory acute leukemia/lymphoma. Clin Cancer Res. 2002;8:1014–1020. [PubMed] [Google Scholar]
- 32.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett J, Childs R. Non-myeloablative stem cell transplants. Br J Haematol. 2000;111:6–17. doi: 10.1046/j.1365-2141.2000.02405.x. [DOI] [PubMed] [Google Scholar]
- 35.Little MT, Storb R. The future of allogeneic hematopoietic stem cell transplantation: minimizing pain, maximizing gain. J Clin Invest. 2000;105:1679–1681. doi: 10.1172/JCI10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Craddock C. Nonmyeloablative stem cell transplants. Curr Opin Hematol. 1999;6:383–387. doi: 10.1097/00062752-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Champlin R, Khouri I, Shimoni A, Gajewski J, Kornblau S, Molldrem J, et al. Harnessing graft-versus-malignancy: non-myeloablative preparative regimens for allogeneic haematopoietic transplantation, an evolving strategy for adoptive immunotherapy. Br J Haematol. 2000;111:18–29. doi: 10.1046/j.1365-2141.2000.02196.x. [DOI] [PubMed] [Google Scholar]
- 38.Champlin R, Khouri I, Kornblau S, Molldrem J, Giralt S. Reinventing bone marrow transplantation: reducing toxicity using nonmyeloablative, preparative regimens and induction of graft-versus-malignancy. Curr Opin Oncol. 1999;11:87–95. doi: 10.1097/00001622-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Lekakis L, de Lima M. Reduced-intensity conditioning and allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:785–798. doi: 10.1586/14737140.8.5.785. [DOI] [PubMed] [Google Scholar]
- 40.Lekakis L, de Padua Silva L, de Lima M. Novel preparative regimens in hematopoietic stem cell transplantation. Curr Pharm Des. 2008;14:1923–1935. doi: 10.2174/138161208785061409. [DOI] [PubMed] [Google Scholar]
- 41.Alchalby H, Kroger N. Reduced-intensity conditioning followed by allogeneic hematopoietic stem cell transplantation in myelofibrosis. Curr Hematol Malig Rep. 2010;5:53–61. doi: 10.1007/s11899-010-0044-z. [DOI] [PubMed] [Google Scholar]
- 42.Couriel DR, Saliba RM, Giralt S, Khouri I, Andersson B, de Lima M, et al. Acute and chronic graft-versus-host disease after ablative and nonmyeloablative conditioning for allogeneic hematopoietic transplantation. Biol Blood Marrow Transplant. 2004;10:178–185. doi: 10.1016/j.bbmt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324:667–674. doi: 10.1056/NEJM199103073241005. [DOI] [PubMed] [Google Scholar]
- 44.Soiffer RJ, Lerademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martino R, Caballero MD, Canals C, San Miguel J, Sierra J, Rovira M, et al. Reduced-intensity conditioning reduces the risk of severe infections after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant. 2001;28:341–347. doi: 10.1038/sj.bmt.1703150. [DOI] [PubMed] [Google Scholar]
- 46.Bornhauser M, Kienast J, Trenschel R, Burchert A, Hegenbart U, Stadler M, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 47.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holowiecki J. Indications for hematopoietic stem cell transplantation. Polskie Archiwum Medycyny Wewnetrznej. 2008;118:658–663. [PubMed] [Google Scholar]
- 49.Pasquini MCWZ Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. 2012 [Google Scholar]
- 50.Farag SS, Maharry K, Zhang MJ, Perez WS, George SL, Mrozek K, et al. Comparison of reduced-intensity hematopoietic cell transplantation with chemotherapy in patients age 60–70 years with acute myelogenous leukemia in first remission. Biol Blood Marrow Transplant. 2011;17:1796–1803. doi: 10.1016/j.bbmt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–1887. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–5738. doi: 10.1200/JCO.2005.15.602. [DOI] [PubMed] [Google Scholar]
- 53.Wong R, Giralt SA, Martin T, Couriel DR, Anagnostopoulos A, Hosing C, et al. Reduced-intensity conditioning for unrelated donor hematopoietic stem cell transplantation as treatment for myeloid malignancies in patients older than 55 years. Blood. 2003;102:3052–3059. doi: 10.1182/blood-2003-03-0855. [DOI] [PubMed] [Google Scholar]
- 54.Taussig DC, Davies AJ, Cavenagh JD, Oakervee H, Syndercombe-Court D, Kelsey S, et al. Durable remissions of myelodysplastic syndrome and acute myeloid leukemia after reduced-intensity allografting. J Clin Oncol. 2003;21:3060–3065. doi: 10.1200/JCO.2003.02.057. [DOI] [PubMed] [Google Scholar]
- 55.Alatrash G, de Lima M, Hamerschlak N, Pelosini M, Wang X, Xiao L, et al. Myeloablative reduced-toxicity i.v. busulfan-fludarabine and allogeneic hematopoietic stem cell transplant for patients with acute myeloid leukemia or myelodysplastic syndrome in the sixth through eighth decades of life. Biol Blood Marrow Transplant. 2011;17:1490–1496. doi: 10.1016/j.bbmt.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura R, Rodriguez R, Palmer J, Stein A, Naing A, Tsai N, et al. Reduced-intensity conditioning for allogeneic hematopoietic stem cell transplantation with fludarabine and melphalan is associated with durable disease control in myelodysplastic syndrome. Bone Marrow Transplant. 2007;40:843–850. doi: 10.1038/sj.bmt.1705801. [DOI] [PubMed] [Google Scholar]
- 57.Blaise DP, Michel Boiron J, Faucher C, Mohty M, Bay JO, Bardoux VJ, et al. Reduced intensity conditioning prior to allogeneic stem cell transplantation for patients with acute myeloblastic leukemia as a first-line treatment. Cancer. 2005;104:1931–1938. doi: 10.1002/cncr.21418. [DOI] [PubMed] [Google Scholar]
- 58.Grigg AP, Gibson J, Bardy PG, Reynolds J, Shuttleworth P, Koelmeyer RL, et al. A prospective multicenter trial of peripheral blood stem cell sibling allografts for acute myeloid leukemia in first complete remission using fludarabine-cyclophosphamide reduced intensity conditioning. Biol Blood Marrow Transplant. 2007;13:560–567. doi: 10.1016/j.bbmt.2006.12.449. [DOI] [PubMed] [Google Scholar]
- 59.Valcarcel D, Martino R, Caballero D, Martin J, Ferra C, Nieto JB, et al. Sustained remissions of high-risk acute myeloid leukemia and myelodysplastic syndrome after reduced-intensity conditioning allogeneic hematopoietic transplantation: chronic graft-versus-host disease is the strongest factor improving survival. J Clin Oncol. 2008;26:577–584. doi: 10.1200/JCO.2007.11.1641. [DOI] [PubMed] [Google Scholar]
- 60.Kohrt HE, Turnbull BB, Heydari K, Shizuru JA, Laport GG, Miklos DB, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohty M, de Lavallade H, Ladaique P, Faucher C, Vey N, Coso D, et al. The role of reduced intensity conditioning allogeneic stem cell transplantation in patients with acute myeloid leukemia: a donor vs no donor comparison. Leukemia. 2005;19:916–920. doi: 10.1038/sj.leu.2403770. [DOI] [PubMed] [Google Scholar]
- 62.Hegenbart U, Niederwieser D, Sandmaier BM, Maris MB, Shizuru JA, Greinix H, et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J Clin Oncol. 2006;24:444–453. doi: 10.1200/JCO.2005.03.1765. [DOI] [PubMed] [Google Scholar]
- 63.Martino R, Iacobelli S, Brand R, Jansen T, van Biezen A, Finke J, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 64.Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott BL, Sandmaier BM, Storer B, Maris MB, Sorror ML, Maloney DG, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 66.van Besien K, Kunavakkam R, Rondon G, De Lima M, Artz A, Oran B, et al. Fludarabine-melphalan conditioning for AML and MDS: alemtuzumab reduces acute and chronic GVHD without affecting long-term outcomes. Biol Blood Marrow Transplant. 2009;15:610–617. doi: 10.1016/j.bbmt.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alyea EP, Kim HT, Ho V, Cutler C, DeAngelo DJ, Stone R, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Ringden O, Labopin M, Ehninger G, Niederwieser D, Olsson R, Basara N, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 69.Shimoni A, Hardan I, Shem-Tov N, Yeshurun M, Yerushalmi R, Avigdor A, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: the role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 70.Flynn CM, Hirsch B, Defor T, Barker JN, Miller JS, Wagner JE, et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: a comparative clinical analysis. Am J Hematol. 2007;82:867–872. doi: 10.1002/ajh.20989. [DOI] [PubMed] [Google Scholar]
- 71.Aoudjhane M, Labopin M, Gorin NC, Shimoni A, Ruutu T, Kolb HJ, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 72.de Lima M, Anagnostopoulos A, Munsell M, Shahjahan M, Ueno N, Ippoliti C, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 73.Gyurkocza B, Storb R, Storer BE, Chauncey TR, Lange T, Shizuru JA, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kantarjian H, Thomas D, O’Brien S, Cortes J, Giles F, Jeha S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–2801. doi: 10.1002/cncr.20668. [DOI] [PubMed] [Google Scholar]
- 75.Takeuchi J, Kyo T, Naito K, Sao H, Takahashi M, Miyawaki S, et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG-ALL93 study. Leukemia. 2002;16:1259–1266. doi: 10.1038/sj.leu.2402526. [DOI] [PubMed] [Google Scholar]
- 76.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 77.Martino R, Giralt S, Caballero MD, Mackinnon S, Corradini P, Fernandez-Aviles F, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica. 2003;88:555–560. [PubMed] [Google Scholar]
- 78.Hamaki T, Kami M, Kanda Y, Yuji K, Inamoto Y, Kishi Y, et al. Reduced-intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant. 2005;35:549–556. doi: 10.1038/sj.bmt.1704776. [DOI] [PubMed] [Google Scholar]
- 79.Mohty M, Labopin M, Tabrizzi R, Theorin N, Fauser AA, Rambaldi A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:303–306. doi: 10.3324/haematol.11960. [DOI] [PubMed] [Google Scholar]
- 80.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113:2902–2905. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]
- 81.Ram R, Storb R, Sandmaier BM, Maloney DG, Woolfrey A, Flowers ME, et al. Non-myeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high-risk acute lymphoblastic leukemia. Haematologica. 2011;96:1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stein AS, Palmer JM, O’Donnell MR, Kogut NM, Spielberger RT, Slovak ML, et al. Reduced-intensity conditioning followed by peripheral blood stem cell transplantation for adult patients with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant. 2009;15:1407–1414. doi: 10.1016/j.bbmt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cho BS, Lee S, Kim YJ, Chung NG, Eom KS, Kim HJ, et al. Reduced-intensity conditioning allogeneic stem cell transplantation is a potential therapeutic approach for adults with high-risk acute lymphoblastic leukemia in remission: results of a prospective phase 2 study. Leukemia. 2009;23:1763–1770. doi: 10.1038/leu.2009.102. [DOI] [PubMed] [Google Scholar]
- 84.Arnold R, Massenkeil G, Bornhauser M, Ehninger G, Beelen DW, Fauser AA, et al. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia. 2002;16:2423–2428. doi: 10.1038/sj.leu.2402712. [DOI] [PubMed] [Google Scholar]
- 85.Eom K-S, Shin S-H, Yoon J-H, Yahng S-A, Lee S-E, Cho B-S, et al. Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol. 2013;88:634–641. doi: 10.1002/ajh.23465. [DOI] [PubMed] [Google Scholar]
- 86.Marks DI, Wang T, Pérez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome–negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 88.Bachanova V, Marks DI, Zhang MJ, Wang H, de Lima M, Aljurf MD, et al. Ph+ ALL patients in first complete remission have similar survival after reduced intensity and myeloablative allogeneic transplantation: impact of tyrosine kinase inhibitor and minimal residual disease. Leukemia. 2013;28:658–665. doi: 10.1038/leu.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das M, Saikia TK, Advani SH, Parikh PM, Tawde S. Use of a reduced-intensity conditioning regimen for allogeneic transplantation in patients with chronic myeloid leukemia. Bone Marrow Transplant. 2003;32:125–129. doi: 10.1038/sj.bmt.1704107. [DOI] [PubMed] [Google Scholar]
- 90.Crawley C, Szydlo R, Lalancette M, Bacigalupo A, Lange A, Brune M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106:2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 91.Luo Y, Lai XY, Tan YM, Shi JM, Zhao YM, Han XY, et al. Reduced-intensity allogeneic transplantation combined with imatinib mesylate for chronic myeloid leukemia in first chronic phase. Leukemia. 2009;23:1171–1174. doi: 10.1038/leu.2008.401. [DOI] [PubMed] [Google Scholar]
- 92.Warlick E, Ahn KW, Pedersen TL, Artz A, de Lima M, Pulsipher M, et al. Reduced intensity conditioning is superior to nonmyeloablative conditioning for older chronic myelogenous leukemia patients undergoing hematopoietic cell transplant during the tyrosine kinase inhibitor era. Blood. 2012;119:4083–4090. doi: 10.1182/blood-2012-02-409763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kebriaei P, Detry MA, Giralt S, Carrasco-Yalan A, Anagnostopoulos A, Couriel D, et al. Long-term follow-up of allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning for patients with chronic myeloid leukemia. Blood. 2007;110:3456–3462. doi: 10.1182/blood-2007-04-085969. [DOI] [PubMed] [Google Scholar]
- 94.Topcuoglu P, Arat M, Ozcan M, Arslan O, Ilhan O, Beksac M, et al. Case-matched comparison with standard versus reduced intensity conditioning regimen in chronic myeloid leukemia patients. Ann Hematol. 2012;91:577–586. doi: 10.1007/s00277-011-1349-2. [DOI] [PubMed] [Google Scholar]
- 95.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 96.Khouri IF, Keating M, Korbling M, Przepiorka D, Anderlini P, O’Brien S, et al. Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol. 1998;16:2817–2824. doi: 10.1200/JCO.1998.16.8.2817. [DOI] [PubMed] [Google Scholar]
- 97.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 98.Ben-Bassat I, Raanani P, Gale RP. Graft-versus-leukemia in chronic lymphocytic leukemia. Bone Marrow Transplant. 2007;39:441–446. doi: 10.1038/sj.bmt.1705619. [DOI] [PubMed] [Google Scholar]
- 99.Pavletic ZS, Arrowsmith ER, Bierman PJ, Goodman SA, Vose JM, Tarantolo SR, et al. Outcome of allogeneic stem cell transplantation for B cell chronic lymphocytic leukemia. Bone Marrow Transplant. 2000;25:717–722. doi: 10.1038/sj.bmt.1702237. [DOI] [PubMed] [Google Scholar]
- 100.Mattsson J, Uzunel M, Remberger M, Ljungman P, Kimby E, Ringden O, et al. Minimal residual disease is common after allogeneic stem cell transplantation in patients with B cell chronic lymphocytic leukemia and may be controlled by graft-versus-host disease. Leukemia. 2000;14:247–254. doi: 10.1038/sj.leu.2401669. [DOI] [PubMed] [Google Scholar]
- 101.Mehta J, Powles R, Singhal S, Iveson T, Treleaven J, Catovsky D, et al. Clinical and hematologic response of chronic lymphocytic and prolymphocytic leukemia persisting after allogeneic bone marrow transplantation with the onset of acute graft-versus-host disease: possible role of graft-versus-leukemia. Bone Marrow Transplant. 1996;17:371–375. [PubMed] [Google Scholar]
- 102.Dreger P. The evolving role of stem cell transplantation in chronic lymphocytic leukemia. Hematol Oncol Clin North Am. 2013;27:355–369. doi: 10.1016/j.hoc.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 103.Sorror ML, Storer BE, Sandmaier BM, Maris M, Shizuru J, Maziarz R, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khouri IF, Bassett R, Poindexter N, O’Brien S, Bueso-Ramos CE, Hsu Y, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011;117:4679–4688. doi: 10.1002/cncr.26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dreger P, Dohner H, Ritgen M, Bottcher S, Busch R, Dietrich S, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 106.Michallet M, Sobh M, Morisset S. Rituximab in allogeneic HSCT for advanced chronic lymphocytic leukemia with fludarabine + total body irradiation conditioning: results of a phase II prospective multicenter study (ITAC 02-02) Bone Marrow Transplant. 2011;46:S43–S44. [Google Scholar]
- 107.Peres E, Braun T, Krijanovski O, Khaled Y, Levine JE, Yanik G, et al. Reduced intensity versus full myeloablative stem cell transplant for advanced CLL. Bone Marrow Transplant. 2009;44:579–583. doi: 10.1038/bmt.2009.61. [DOI] [PubMed] [Google Scholar]
- 108.Brown JR, Kim HT, Armand P, Cutler C, Fisher DC, Ho V, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27:362–369. doi: 10.1038/leu.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Giralt S, Aleman A, Anagnostopoulos A, Weber D, Khouri I, Anderlini P, et al. Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplant. 2002;30:367–373. doi: 10.1038/sj.bmt.1703652. [DOI] [PubMed] [Google Scholar]
- 110.Mohty M, Boiron JM, Damaj G, Michallet AS, Bay JO, Faucher C, et al. Graft-versus-myeloma effect following antithymocyte globulin-based reduced intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:77–84. doi: 10.1038/sj.bmt.1704531. [DOI] [PubMed] [Google Scholar]
- 111.Gerull S, Goerner M, Benner A, Hegenbart U, Klein U, Schaefer H, et al. Long-term outcome of nonmyeloablative allogeneic transplantation in patients with high-risk multiple myeloma. Bone Marrow Transplant. 2005;36:963–969. doi: 10.1038/sj.bmt.1705161. [DOI] [PubMed] [Google Scholar]
- 112.Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H, et al. Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplantation-related mortality. Blood. 2002;100:3919–3924. doi: 10.1182/blood-2002-04-1150. [DOI] [PubMed] [Google Scholar]
- 113.Crawley C, Iacobelli S, Bjorkstrand B, Apperley JF, Niederwieser D, Gahrton G, et al. Reduced-intensity conditioning for myeloma: lower nonrelapse mortality but higher relapse rates compared with myeloablative conditioning. Blood. 2007;109:3588–3594. doi: 10.1182/blood-2006-07-036848. [DOI] [PubMed] [Google Scholar]
- 114.Koreth J, Stevenson KE, Kim HT, Garcia M, Ho VT, Armand P, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–3959. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khouri IF, Saliba RM, Erwin WD, Samuels BI, Korbling M, Medeiros LJ, et al. Nonmyeloablative allogeneic transplantation with or without 90yttrium ibritumomab tiuxetan is potentially curative for relapsed follicular lymphoma: 12-year results. Blood. 2012;119:6373–6378. doi: 10.1182/blood-2012-03-417808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piñana JL, Martino R, Gayoso J, Sureda A, de la Serna J, Díez-Martín JL, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vigouroux S, Michallet M, Porcher R, Attal M, Ades L, Bernard M, et al. Long-term outcomes after reduced-intensity conditioning allogeneic stem cell transplantation for low-grade lymphoma: a survey by the French Society of Bone Marrow Graft Transplantation and Cellular Therapy (SFGM-TC) Haematologica. 2007;92:627–634. doi: 10.3324/haematol.10924. [DOI] [PubMed] [Google Scholar]
- 119.Cook G, Smith GM, Kirkland K, Lee J, Pearce R, Thomson K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2010;16:1419–1427. doi: 10.1016/j.bbmt.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 120.Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: a multicentre experience. Br J Haematol. 2008;143:395–403. doi: 10.1111/j.1365-2141.2008.07365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thomson KJ, Morris EC, Bloor A, Cook G, Milligan D, Parker A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–432. doi: 10.1200/JCO.2008.17.3328. [DOI] [PubMed] [Google Scholar]
- 122.Sirvent A, Dhedin N, Michallet M, Mounier N, Faucher C, Yakoub-Agha I, et al. Low nonrelapse mortality and prolonged long-term survival after reduced-intensity allogeneic stem cell transplantation for relapsed or refractory diffuse large B cell lymphoma: report of the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Biol Blood Marrow Transplant. 2010;16:78–85. doi: 10.1016/j.bbmt.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 123.Hari P, Carreras J, Zhang MJ, Gale RP, Bolwell BJ, Bredeson CN, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hamadani M, Saber W, Ahn KW, Carreras J, Cairo MS, Fenske TS, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: a cohort analysis from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2013;19:625–631. doi: 10.1016/j.bbmt.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bacher U, Klyuchnikov E, Le-Rademacher J, Carreras J, Armand P, Bishop MR, et al. Conditioning regimens for allotransplants for diffuse large B-cell lymphoma: myeloablative or reduced intensity? Blood. 2012;120:4256–4262. doi: 10.1182/blood-2012-06-436725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomson KJ, Morris EC, Milligan D, Parker AN, Hunter AE, Cook G, et al. T-cell-depleted reduced-intensity transplantation followed by donor leukocyte infusions to promote graft-versus-lymphoma activity results in excellent long-term survival in patients with multiply relapsed follicular lymphoma. J Clin Oncol. 2010;28:3695–3700. doi: 10.1200/JCO.2009.26.9100. [DOI] [PubMed] [Google Scholar]
- 127.Shea T, Johnson J, Westervelt P, Farag S, McCarty J, Bashey A, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hamadani M, Saber W, Ahn KW, Carreras J, Cairo MS, Fenske TS, et al. Allogeneic hematopoietic cell transplantation for chemotherapy-unresponsive mantle cell lymphoma: A Cohort Analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2013;19:625–631. doi: 10.1016/j.bbmt.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wei W, Valverde R, Korbling M, Turturro F, Gulbis AM, Guillermo-Pacheco M, et al. Bfr (bendamustine, fludarabine, rituximab) nonmyeloablative allogeneic conditioning: a novel regimen inducing immunosuppression without myelosuppression. Blood. 2013;122:541. [Google Scholar]
- 130.Gopal AK, Guthrie KA, Rajendran J, Pagel JM, Oliveira G, Maloney DG, et al. (9)(0) Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118:1132–1139. doi: 10.1182/blood-2010-12-324392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alvarez I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M, et al. Nonmyeloablative stem cell transplantation is an effective therapy for refractory or relapsed hodgkin lymphoma: results of a spanish prospective cooperative protocol. Biol Blood Marrow Transplant. 2006;12:172–183. doi: 10.1016/j.bbmt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 132.Sureda A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma. Results of the HDR-ALLO study -a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sureda A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, et al. Reduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2008;26:455–462. doi: 10.1200/JCO.2007.13.2415. [DOI] [PubMed] [Google Scholar]
- 134.Anderlini P, Saliba R, Acholonu S, Okoroji GJ, Donato M, Giralt S, et al. Reduced-intensity allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s disease: low transplant-related mortality and impact of intensity of conditioning regimen. Bone Marrow Transplant. 2005;35:943–951. doi: 10.1038/sj.bmt.1704942. [DOI] [PubMed] [Google Scholar]
- 135.Anderlini P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT, et al. Fludarabine-melphalan as a preparative regimen for reduced-intensity conditioning allogeneic stem cell transplantation in relapsed and refractory Hodgkin’s lymphoma: the updated M.D. Anderson Cancer Center experience. Haematologica. 2008;93:257–264. doi: 10.3324/haematol.11828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robinson SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for Hodgkin’s lymphoma: identification of prognostic factors predicting outcome. Haematologica. 2009;94:230–238. doi: 10.3324/haematol.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anderlini P, Saliba RM, Ledesma C, Chancoco CM, Alexander T, Alousi A, et al. Reduced-intensity conditioning (RIC) and allogeneic stem cell transplantation (allo-SCT) for relapsed/refractory Hodgkin lymphoma (HL) in the Brentuximab Vedotin era: favorable overall and progression-free survival (OS/PFS) with low transplant-related mortality (TRM) Blood. 2013;122:410. [Google Scholar]
- 138.Giralt S, Thall PF, Khouri I, Wang X, Braunschweig I, Ippolitti C, et al. Melphalan and purine analog–containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97:631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 139.Bryant A, Nivison-Smith I, Pillai ES, Kennedy G, Kalff A, Ritchie D, et al. Fludarabine Melphalan reduced-intensity conditioning allotransplanation provides similar disease control in lymphoid and myeloid malignancies: analysis of 344 patients. Bone Marrow Transplant. 2014;49:17–23. doi: 10.1038/bmt.2013.142. [DOI] [PubMed] [Google Scholar]
- 140.Chakraverty R, Peggs K, Chopra R, Milligan DW, Kottaridis PD, Verfuerth S, et al. Limiting transplantation-related mortality following unrelated donor stem cell transplantation by using a nonmyeloablative conditioning regimen. Blood. 2002;99:1071–1078. doi: 10.1182/blood.v99.3.1071. [DOI] [PubMed] [Google Scholar]
- 141.Hale G, Zhang MJ, Bunjes D, Prentice HG, Spence D, Horowitz MM, et al. Improving the outcome of bone marrow transplantation by using CD52 monoclonal antibodies to prevent graft-versus-host disease and graft rejection. Blood. 1998;92:4581–4590. [PubMed] [Google Scholar]
- 142.Perez-Simon JA, Kottaridis PD, Martino R, Craddock C, Caballero D, Chopra R, et al. Nonmyeloablative transplantation with or without alemtuzumab: comparison between 2 prospective studies in patients with lymphoproliferative disorders. Blood. 2002;100:3121–3127. doi: 10.1182/blood-2002-03-0701. [DOI] [PubMed] [Google Scholar]
- 143.Soiffer RJ, LeRademacher J, Ho V, Kan F, Artz A, Champlin RE, et al. Impact of immune modulation with anti–T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood. 2011;117:6963–6970. doi: 10.1182/blood-2011-01-332007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–2468. doi: 10.1038/leu.2012.135. [DOI] [PubMed] [Google Scholar]
- 145.Gartner F, Hieke S, Finke J, Bertz H. Lowering the alemtuzumab dose in reduced intensity conditioning allogeneic hematopoietic cell transplantation is associated with a favorable early intense natural killer cell recovery. Cytotherapy. 2013;15:1237–1244. doi: 10.1016/j.jcyt.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 146.Brunner AM, Kim HT, Coughlin E, Alyea EP, 3rd, Armand P, Ballen KK, et al. Outcomes in patients age 70 or older undergoing allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2013;19:1374–1380. doi: 10.1016/j.bbmt.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 147.Chen YB, Coughlin E, Kennedy KF, Alyea EP, Armand P, Attar EC, et al. Busulfan dose intensity and outcomes in reduced-intensity allogeneic peripheral blood stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia. Biol Blood Marrow Transplant. 2013;19:981–987. doi: 10.1016/j.bbmt.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 148.Devillier R, Furst S, El-Cheikh J, Castagna L, Harbi S, Granata A, et al. Antithymocyte globulin in reduced-intensity conditioning regimen allows a high disease-free survival exempt of long-term chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:370–374. doi: 10.1016/j.bbmt.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 149.Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–1883. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kornblit B, Maloney DG, Storb R, Storek J, Hari P, Vucinic V, et al. Fludarabine and 2-Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: a phase III randomized trial. Biol Blood Marrow Transplant. 2013;19:1340–1347. doi: 10.1016/j.bbmt.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Michallet M, Sobh M, Milpied N, Bay JO, Furst S, Harousseau JL, et al. Phase II prospective study of treosulfan-based reduced-intensity conditioning in allogeneic HSCT for hematological malignancies from 10/10 HLA-identical unrelated donor. Ann Hematol. 2012;91:1289–1297. doi: 10.1007/s00277-012-1429-y. [DOI] [PubMed] [Google Scholar]
- 152.Buchholz S, Dammann E, Stadler M, Krauter J, Beutel G, Trummer A, et al. Cytoreductive treatment with clofarabine/ara-C combined with reduced-intensity conditioning and allogeneic stem cell transplantation in patients with high-risk, relapsed, or refractory acute myeloid leukemia and advanced myelodysplastic syndrome. Eur J Haematol. 2012;88:52–60. doi: 10.1111/j.1600-0609.2011.01703.x. [DOI] [PubMed] [Google Scholar]
- 153.Chevallier P, Labopin M, Socie G, Tabrizi R, Furst S, Lioure B, et al. Results from a clofarabine-busulfan-containing, reduced-toxicity conditioning regimen prior to allogeneic stem cell transplantation: the phase 2 prospective CLORIC trial. Haematologica. 2014;99:1486–1491. doi: 10.3324/haematol.2014.108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kirschbaum MH, Stein AS, Popplewell L, Delioukina M, Chen R, Nakamura R, et al. A phase I study in adults of clofarabine combined with high-dose melphalan as reduced-intensity conditioning for allogeneic transplantation. Biol Blood Marrow Transplant. 2012;18:432–440. doi: 10.1016/j.bbmt.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 155.Chevallier P, Labopin M, Buchholz S, Ganser A, Ciceri F, Lioure B, et al. Clofarabine-containing conditioning regimen for allo-SCT in AML/ALL patients: a survey from the Acute Leukemia Working Party of EBMT. Eur J Haematol. 2012;89:214–219. doi: 10.1111/j.1600-0609.2012.01822.x. [DOI] [PubMed] [Google Scholar]
- 156.Pagel JM, Gooley TA, Rajendran J, Fisher DR, Wilson WA, Sandmaier BM, et al. Allogeneic hematopoietic cell transplantation after conditioning with 131I–anti-CD45 antibody plus fludarabine and low-dose total body irradiation for elderly patients with advanced acute myeloid leukemia or high-risk myelodysplastic syndrome. Blood. 2009;114:5444–5453. doi: 10.1182/blood-2009-03-213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ringhoffer M, Blumstein N, Neumaier B, Glatting G, von Harsdorf S, Buchmann I, et al. 188Re or 90Y-labelled anti-CD66 antibody as part of a dose-reduced conditioning regimen for patients with acute leukaemia or myelodysplastic syndrome over the age of 55: results of a phase I–II study. Br J Haematol. 2005;130:604–613. doi: 10.1111/j.1365-2141.2005.05663.x. [DOI] [PubMed] [Google Scholar]
- 158.McDevitt MR, Sgouros G, Finn RD, Humm JL, Jurcic JG, Larson SM, et al. Radioimmunotherapy with alpha-emitting nuclides. Eur J Nucl Med. 1998;25:1341–1351. doi: 10.1007/s002590050306. [DOI] [PubMed] [Google Scholar]
- 159.Jurcic JG, Rosenblat TL. Targeted alpha-particle immunotherapy for acute myeloid leukemia. Am Soc Clin Oncol Educ Book. 2014:e126–e131. doi: 10.14694/EdBook_AM.2014.34.e126. [DOI] [PubMed] [Google Scholar]
- 160.Jurcic JG, Larson SM, Sgouros G, McDevitt MR, Finn RD, Divgi CR, et al. Targeted alpha particle immunotherapy for myeloid leukemia. Blood. 2002;100:1233–1239. [PubMed] [Google Scholar]
- 161.Rosenblat TL, McDevitt MR, Mulford DA, Pandit-Taskar N, Divgi CR, Panageas KS, et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res. 2010;16:5303–5311. doi: 10.1158/1078-0432.CCR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sandmaier BM, Bethge WA, Wilbur DS, Hamlin DK, Santos EB, Brechbiel MW, et al. Bismuth 213-labeled anti-CD45 radioimmunoconjugate to condition dogs for nonmyeloablative allogeneic marrow grafts. Blood. 2002;100:318–326. doi: 10.1182/blood-2001-12-0322. [DOI] [PubMed] [Google Scholar]
- 163.Bethge WA, Wilbur DS, Storb R, Hamlin DK, Santos EB, Brechbiel MW, et al. Selective T-cell ablation with bismuth-213-labeled anti-TCRalphabeta as non-myeloablative conditioning for allogeneic canine marrow transplantation. Blood. 2003;101:5068–5075. doi: 10.1182/blood-2002-12-3867. [DOI] [PubMed] [Google Scholar]
- 164.Pagel JM, Kenoyer AL, Back T, Hamlin DK, Wilbur DS, Fisher DR, et al. Anti-CD45 pretargeted radioimmunotherapy using bismuth-213: high rates of complete remission and long-term survival in a mouse myeloid leukemia xenograft model. Blood. 2011;118:703–711. doi: 10.1182/blood-2011-04-347039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sharkey RM, Karacay H, Chang CH, McBride WJ, Horak ID, Goldenberg DM, et al. Improved therapy of non-Hodgkin’s lymphoma xenografts using radionuclides pretargeted with a new anti-CD20 bispecific antibody. Leukemia. 2005;19:1064–1069. doi: 10.1038/sj.leu.2403751. [DOI] [PubMed] [Google Scholar]
- 166.Liu G, Dou S, Mardirossian G, He J, Zhang S, Liu X, et al. Successful radiotherapy of tumor in pretargeted mice by 188Re-radiolabeled phosphorodiamidate morpholino oligomer, a synthetic DNA analogue. Clin Cancer Res. 2006;12:4958–4964. doi: 10.1158/1078-0432.CCR-06-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chargari C, Vernant JP, Tamburini J, Zefkili S, Fayolle M, Campana F, et al. Feasibility of helical tomotherapy for debulking irradiation before stem cell transplantation in malignant lymphoma. Int J Radiat Oncol Biol Phys. 2011;81:1184–1189. doi: 10.1016/j.ijrobp.2010.01.051. [DOI] [PubMed] [Google Scholar]


