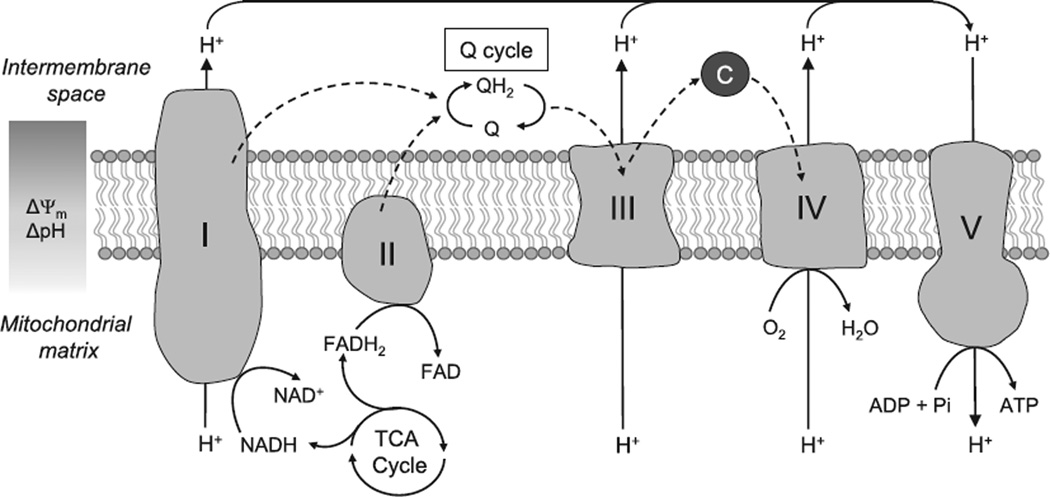
Figure 1. The mitochondrial electron transport chain.
Electrons derived from the oxidation of NADH and FADH2 enter the electron transport chain at complexes I (NADH ubiquinone oxidoreductase) and II (Succinate dehydrogenase). They are then funneled through the electron carriers, Coenzyme Q, and complex III (Ubiquinol cytochrome c oxidoreductase), until they reach complex IV (cytochrome c oxidase) where they are used to reduce molecular oxygen to water. This transfer of electrons is coupled to the extrusion of protons at complexes I, III, and IV generating an electrochemical gradient across the mitochondrial membrane. Protons in the intermembrane space are then used to drive the synthesis of ATP at complex V (ATP synthase). C indicates cytochrome c; and TCA, tricarboxylic acid. Dashed arrows indicate path of electrons.