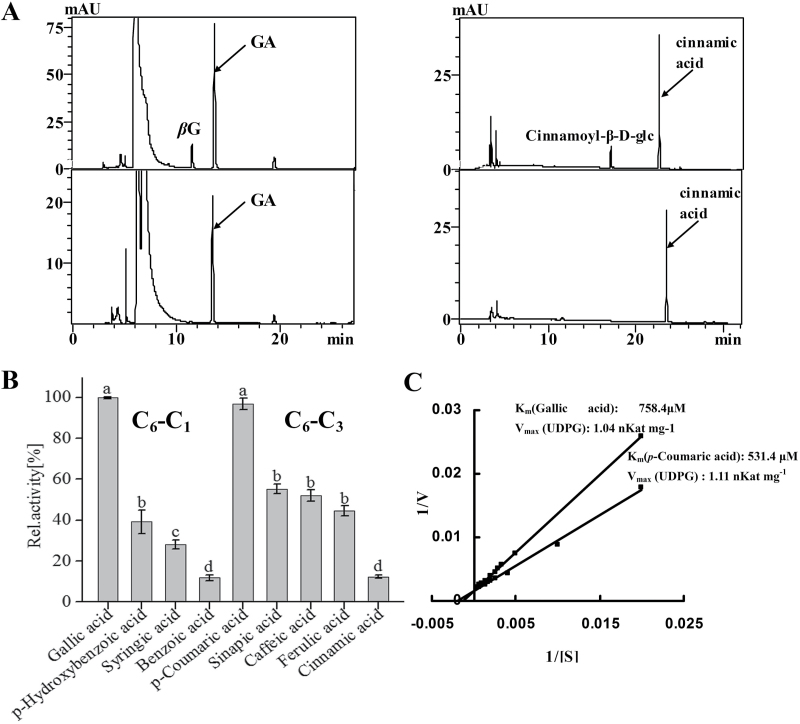
Fig. 3.
Enzymatic analysis of the recombinant UGT proteins with different substrates. (A) Representative HPLC chromatograms for enzymatic reactions with substrates gallic acid (left) and cinnamic acid (right) and their corresponding control reactions (lower panels). (B) The relative activity of CsUGT84A22 with different substrates. Assays were performed with 2mM of benzoic acids (C6–C1) or cinnamic acids (C6–C3) as acceptors and 2.5mM UDP-Glc as the sugar donor. Relative activity was referred to the reaction with gallic acid as the substrate (100%). All data presented here are the means of three replicates. Different letters above the bars indicate statistically significant differences at P <0.05, based on Tukey’s honestly significant difference test. (C) A double reciprocal plot showing 1/V versus 1/[gallic acid] and 1/V versus 1/[coumaric acid]. K m and V max values for recombinant CsUGT84A22 protein for these substrates are listed in the plot. All data presented are the means of three independent replicates.