Fig. 1.
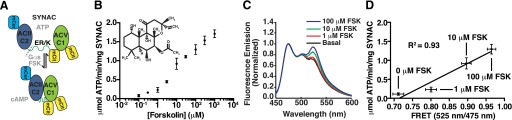
Characterization of synthetic adenylyl cyclase (SYNAC). (A) Schematic diagram of SYNAC. Black dashed line indicates TEV protease cleavage site. Schematic shows the expected complementation of the ACII-C2 and ACV-C1 domains after stimulation with activated GαS (GTPγS) or FSK. (B) SYNAC displays FSK-stimulated activity in a concentration-dependent fashion. Activity is measured in terms of ATP metabolized by SYNAC. Inset is the structure of FSK. Data are mean ± S.E.M. of three independent repeats using three different preparations of protein. (C) FSK stimulates domain complementation in SYNAC. Normalized fluorescence emission spectrum (mCerulean, excitation 430 nm) of SYNAC stimulated with the indicated concentrations of FSK. Data are the average of three repeats. (D) FRET of SYNAC (mCit/mCer; 525 nm/475 nm) plotted against activity at the indicated concentrations of the compound. R2 of 0.93 indicates a linear relationship between the two parameters. Activity data are mean ± S.E.M. of three independent repeats using three different preparations of protein. FRET data are mean ± S.D. of three spectra. Spectra are presented as mean without error bars for clarity.