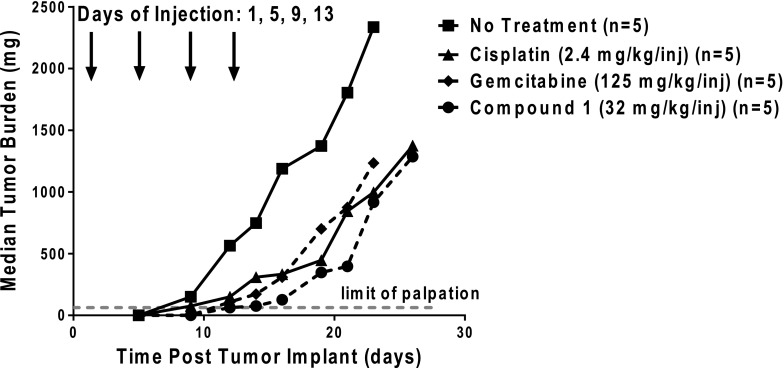
Fig. 6.
Analysis of in vivo efficacy of C1. An in vivo efficacy trial of C1 in H460 xenografts was performed. Female ICR SCID mice were maintained on a folate-deficient diet ad libitum. Human H460 tumors were implanted bilaterally and subcutaneously, and mice were nonselectively randomized into five mice per group. C1 [32 mg/kg injection, dissolved in 5% ethanol (v/v), 1% Tween 80 (v/v), and 0.5% NaHCO3], gemcitabine (125 mg/kg injection, dissolved in 0.9% saline), and cisplatin (2.4 mg/kg injection, dissolved in 0.9% saline) were administered on a schedule of every 4 days for four intravenous treatments on days 1, 5, 9, and 13 (indicated by arrows). Mice were observed and weighed daily; tumors were measured 2 to 3 times per week. On day 16, T/C values equaled 11%, 26%, and 28% for C1, gemcitabine, and cisplatin, respectively. Antitumor activities were recorded for C1 (T–C = 9 days; 1.9 gross log10 kill), gemcitabine (T–C = 7 days; 1.5 gross log10 kill), and cisplatin (T–C = 8 days, 1.7 gross log10 kill). Data are shown for the median tumor burdens of each treatment group.