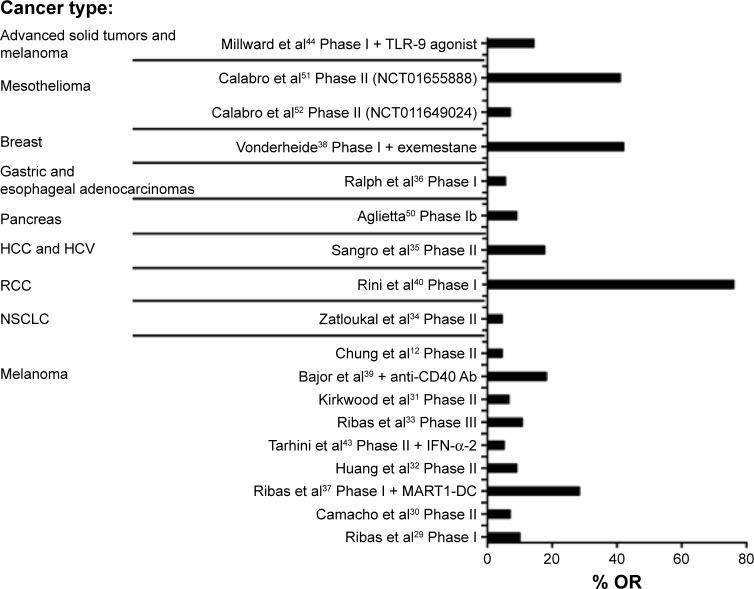
Figure 2.
Overall objective response rate or overall response (OR) to treatment with tremelimumab in different cancer types.
Notes: Clinical benefit expressed as overall objective response or overall response (OR) of all the tremelimumab clinical trials. The percentage of OR is expressed on X-axis; and all the tremelimumab clinical trials that presented a clinical response are represented on the Y-axis. The high percentage of OR is due to the low number of patients normally enrolled on those Phase I clinical trials.
Abbreviations: DC, dendritic cell; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IFN-α-2, interferon-α-2; NSCLC, non-small-cell lung cancer; RCC, renal cell carcinoma; TLR-9, Toll-like receptor-9; MART-1, melanoma antigen recognized by T-cells.