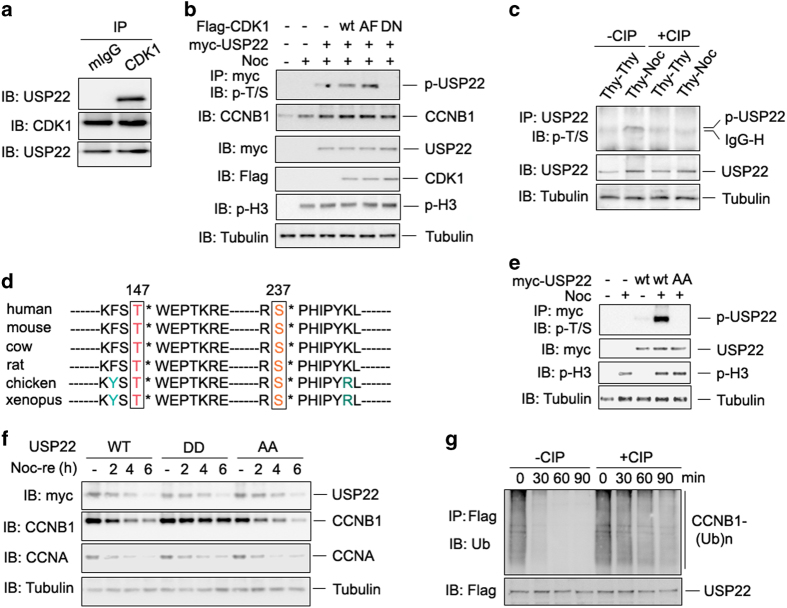
Figure 4.
Cyclin-dependent kinase 1 (CDK1) phosphorylates ubiquitin-specific protease 22 (USP22) to promote its deubiquitinase activity. (a) The endogenous interaction between CDK1 and USP22 was analyzed as in Figure 2b. (b) Myc-USP22 plasmids were co-expressed with wild-type (WT), the constitutively active form (AF) of CDK1, or the kinase-inactive D146N mutant of CDK1. After 24 h, cells were treated without or with nocodazole for 12 h. USP22 phosphorylation in the lysates of transfected cells was analyzed. (c) HCT116 cells were treated with double thymidine (Thy–Thy) or with thymidine followed by nocodazole (Thy–Noc). The cell lysates were treated with or without calf intestinal alkaline phosphatase (CIP) for 1 h as indicated. USP22 phosphorylation in the lysates of treated cells was analyzed as described in b. (d) Conserved T147 and S237 amino acids of USP22 in each indicated species are shown. (e) WT USP22 or its phosphorylation-defective mutant USP22/AA was co-expressed in HCT116 cells. Their phosphorylation was determined as in b. (f) HCT116 cells stably expressing WT USP22 or its phosphomimetic mutant (USP22/DD) or phosphorylation-defective mutant USP22/AA were synchronized in prometaphase with Thy and Noc treatment, then released into fresh medium for the indicated times (Noc-re). (g) HA-ubiquitin-conjugated Flag-CCNB1 was affinity purified from co-transfected HCT116 cells. Flag-USP22 was purified from HCT116 cells and treated with or without CIP for 1 h. Ubiquitinated CCNB1 was then mixed with USP22 for indicated time and analyzed by immunoblotting.