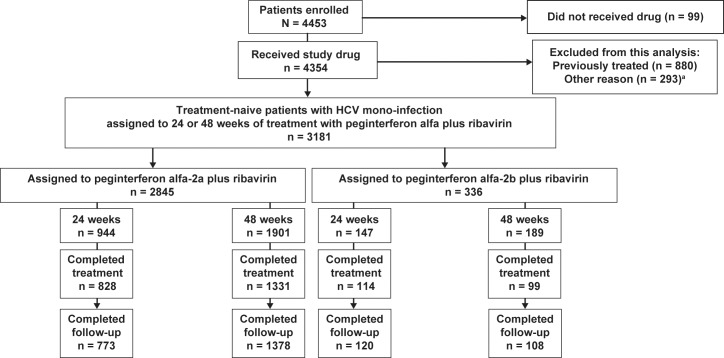
Fig 1. Enrollment and patient disposition.
aOther reasons (more than one reason may apply to a given patient): no final confirmation from the investigator (n = 56); contraindications to therapy (n = 15); HCV RNA-negative at screening/baseline (n = 12); end-stage renal disease (n = 7); major organ transplantation (n = 2); not treated with peginterferon alfa (n = 1) or ribavirin (n = 2); acute hepatitis C (n = 1); co-infection with HIV (n = 115); co-infection with HBV (n = 74); treatment with regimen other than peginterferon alfa-2a/ribavirin or peginterferon alfa-2b/ribavirin (n = 14); treatment-naive and intended treatment duration of 72 weeks (n = 6).