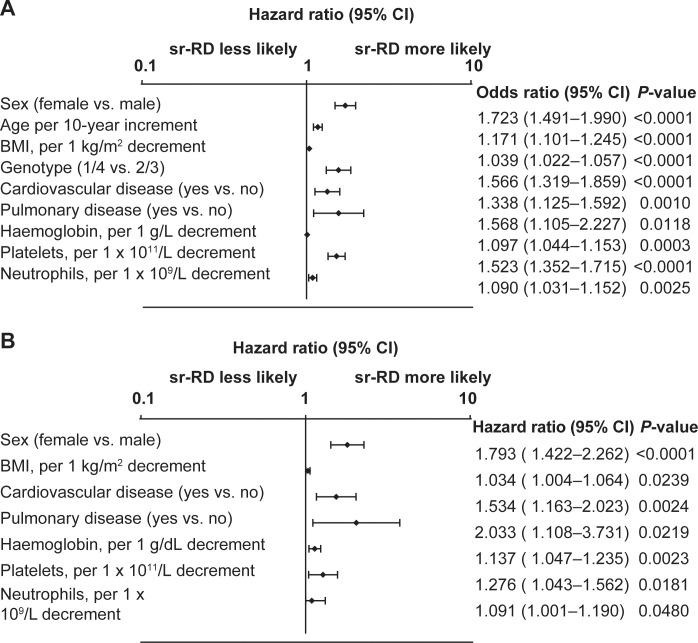
Fig 2. Cox proportional hazards analysis for time to first safety-related dose reductions or discontinuations in patients treated for 24 or 48 weeks with peginterferon alfa-2a or alfa-2b and ribavirin.
(A) All treatment-naive patients (G1–6) assigned to 24 or 48 weeks of treatment with peginterferon alfa-2a or alfa-2b/RBV (N = 3181); (B) Subgroup 2: treatment-naive Caucasian, G1 noncirrhotic patients assigned to 48 weeks of treatment with peginterferon alfa-2a/RBV (n = 951).