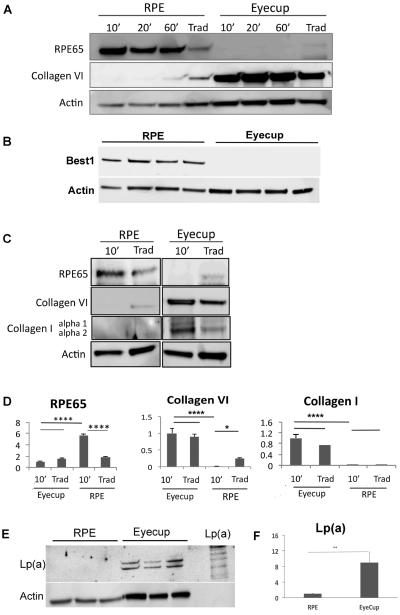
Figure 5.
Protein recovery by incubation time in C57BL/6x129 mice. RPE protein were collected after removing retina and choroid/sclera, incubated in protein lysis buffer for 10 min, 20min, and 60 min, and compared to protein recovered using the traditional method (Trad) that contained RPE-Bruch's membrane-choroidal proteins (n=5 each group). A. The lysis buffer incubation protocol results in abundant RPE 65, an RPE specific gene, and minimal choroidal contamination, as confirmed by low Collagen VI in RPE lysates. In comparison, collagen VI is abundant in the RPE/choroidal extracts using the traditional method. RPE65 is absent from the extracts recovered from the remaining eyecup after both the lysis buffer and traditional protocols. Collagen VI was abundant in the remaining eyecups after both the lysis buffer and traditional protocols. B. Western blot of RPE and eyecup lysates after a 10 minute incubation in lysis buffer showing Best1 in the RPE lysate, but not in the remaining eyecup (n=4 mice). C. Representative Western blot comparing a 10 minute incubation in lysis buffer to the traditional dissection of both the RPE lysates and of the remaining eyecup after dissection, for RPE65, collagen VI, and collagen I. Expression was normalized to actin. Note that the actin abundance is different between the RPE and eyecup. D. Graph showing the quantification of RPE65, collagen VI, and collagen I from the Western blots in C (n=5 mice). *p<0.05, ***p<0.001, ****p<0.0001. E. Western blot of Lp(a) using LPA4 antibody against human apo(a) in the RPE and remaining eyecup using the lysis buffer protocol (n=3 mice per group). An aliquot of Lp(a) is shown for comparison to illustrate the multiple bands which identify Lp(a). F. Graph quantifying the abundance of Lp(a) in the RPE and eycup lysates; **p<0.01.