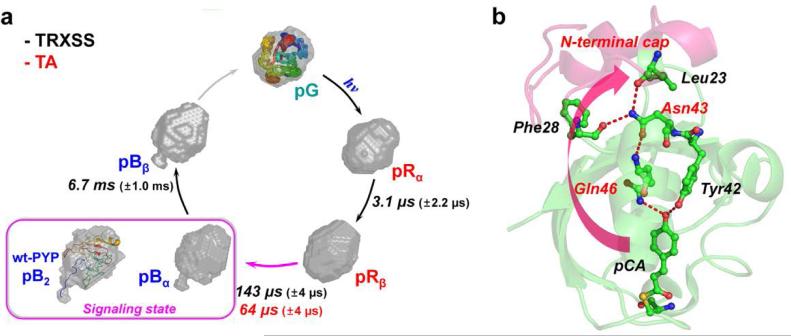
Fig. 4.
Structural dynamics of E46Q-PYP photocycle and hydrogen-bonding network from the chromophore binding pocket to the N-terminal cap. (a) Global kinetics of E46Q-PYP photocycle. The reconstructed molecular shapes of the intermediates were extracted from the structural analysis of the scattering data. The photocycle of E46Q-PYP includes four intermediates (pRα, pRβ, pBα, and pBβ) and the transition rates among the intermediates were determined from time-resolved X-ray solution scattering (TRXSS) and transient absorption spectroscopy (TA). The global conformational change of the protein is maximal in the pRβ → pBα transition occurring on the time scale of 143 μs as supported by the reconstructed shape from TRXSS. The inset (magenta box) shows the reconstructed molecular shape of the signaling state (pB2) of wt-PYP14 with the atomistic structure determined from the previous study combining DEER, NMR, and SAXS/WAXS experiment.51 (b) The local structural change in the vicinity of chromophore (pCA) can be propagated up to the N-terminal cap region through the hydrogen-bonding network (red dashed lines). The weak hydrogen bond between pCA and Gln46 in E46Q PYP may result in the smaller global conformational change compared with the change in wild-type PYP.