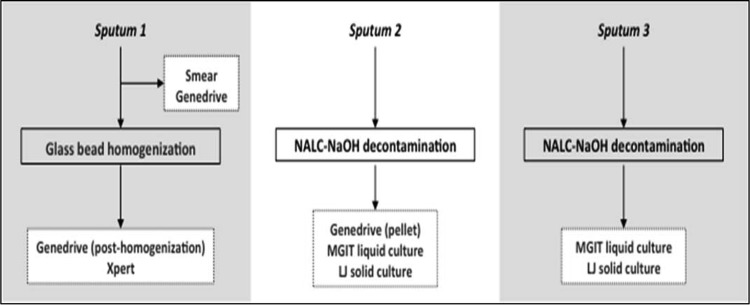
FIG 1.
Specimen processing and testing schema for the prospective multicenter diagnostic accuracy clinical study. Each participant provided 3 sputum samples. Sputum 1 was used to perform direct smear microscopy and the Genedrive assay before being homogenized with glass beads. After homogenization, it was split into 2 portions for testing with the Genedrive assay and the Xpert MTB/RIF assay. Sputum 2 was decontaminated by the standard NALC-NaOH method, and then the resuspended pellet was used to perform liquid MGIT and solid Lowenstein-Jensen (LJ) cultures and the Genedrive assay. Sputum 3 was decontaminated by the standard NALC-NaOH method, and then the resuspended pellet was used to perform MGIT and LJ cultures.