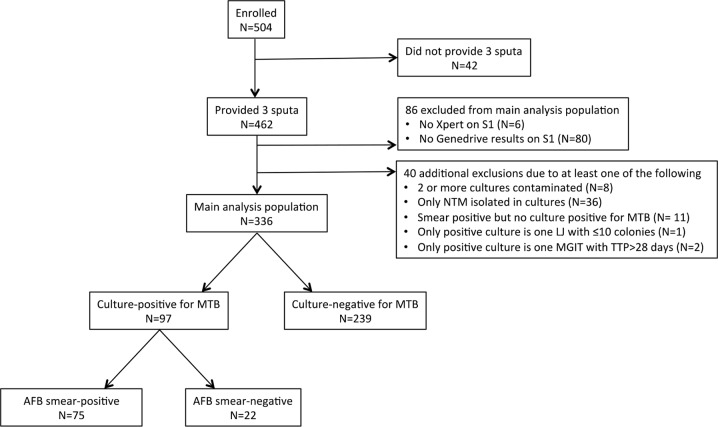
FIG 3.
Overview of patient enrollment and study outcomes. A total 504 suspected pulmonary TB patients were recruited in this study, of which 42 patients were unable to produce sputum specimens. Of the remaining 462, 80 participants did not have a determinate Genedrive result on sputum 1 (12 with test result of “error” and 68 with Genedrive testing not performed due to instrument inoperability or no intact cartridges at the testing site), and 6 participants did not have a determinate Xpert result for sputum 1 (2 with test result of ‘error’ and 4 with Xpert testing not performed due to human error) and hence were excluded from the study. Of the 336 participants who completed this study, 97 were culture positive and 239 were culture negative. Among culture positives, 75 were AFB positive and 22 were AFB negative by smear microscopy. S1, sputum 1; NTM, nontuberculous mycobacteria; MTB, M. tuberculosis; LJ, Lowenstein-Jensen culture; MGIT, mycobacterial growth indicator tube culture; TTP, time to positivity; AFB, acid-fast bacilli.