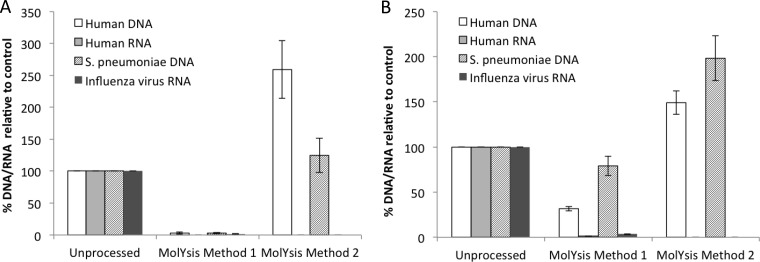
FIG 1.
Processing of clinical specimens using MolYsis reagents. CSF (A) or NPA (B) specimens were spiked with S. pneumoniae and influenza A virus and processed by MolYsis method I and MolYsis method II, as described in the Materials and Methods. Nucleic acids extracted from processed or unprocessed samples were analyzed by real-time PCR assays for β-actin DNA and β-2-microglobulin RNA for human DNA and RNA, respectively, and pathogen-specific targets, as described in Table 1. The percentage of DNA/RNA was obtained from fold changes calculated from CT values, with respect to CT values for unprocessed samples. The data are the average of data obtained from 3 independent experiments using a total of 3 CSF specimens and 3 NPA specimens. Error bars are the standard error of the mean; statistical significance was calculated by the pairwise Student's t test (two-tailed).