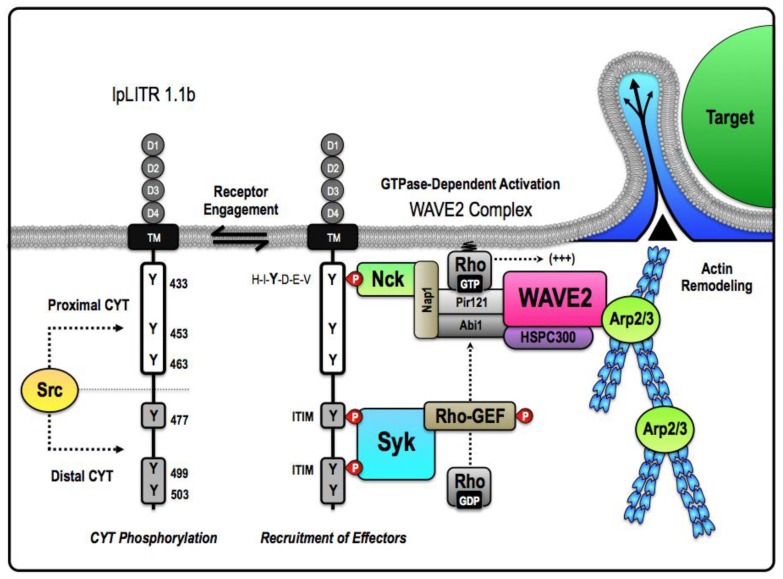
Figure 2.
Proposed mechanism for an ITAM-independent target acquisition and engulfment pathway facilitated by pLITR 1.1b. The unique short-circuited version of phagocytosis exhibited by IpLITR 1.1b-expressing cells likely requires that the proximal and distal regions of the IpLITR 1.1b CYT differentially participate in the recruitment and activation of select phagocytic effectors. Our results suggest that IpLITR 1.1b-mediated regulation of the actin polymerization machinery is dependent upon the catalytic activity of the Src and spleen tyrosine kinase (Syk) families of intracellular kinases [57]. We hypothesize that Src serves to place IpLITR 1.1b in a primed state that facilitates basal or constitutive coupling of IpLITR 1.1b to the minimal intracellular machinery required for target acquisition and phagocytic cup extension. In this model, the cytosolic adaptor non-catalytic region of tyrosine kinase adaptor protein 1 (Nck) is recruited to the consensus interaction motif H-I-Y-D-E-V located at Y433 in the proximal CYT region of IpLITR 1.1b. Nck has been shown to associate with the WAVE2 complex; a highly conserved pentameric heterocomplex that dynamically regulates Arp2/3-dependent actin polymerization [104]. Importantly, in mammalian cells, WAVE2 is expressed ubiquitously and found as a complex with four other proteins: Pir121, Nap1, Abi-1, and HSPC300 [104]. The mature WAVE2 complex is basally inactive and directly interacts with the SH3 domain of Nck through Nap1 [103,105]. Activation of the WAVE2 complex requires state-specific phosphorylation as well as interactions with GTP-bound Rho superfamily proteins, most commonly Rac [106]. As a result, we propose that the assembly of the Nck-WAVE2 complex within the proximal CYT region of IpLITR 1.1b could be coupled to recruitment of a stimulatory Rho-GEF within the distal CYT region. In particular, the spacing of the tyrosines in IpLITR 1.1b suggest that Syk could be recruited to two tandem ITIM motifs at Y477 and Y499 in the distal CYT region. Based on comparisons with other phagocytic receptors, we suspect that activation of the cytosolic Rho-GEF could be Syk-dependent. Syk activation of the Rho-GEF would provide the necessary catalyst for rapid actin-driven membrane protrusions via the WAVE2 complex. Together, this mechanism would encompass the minimal machinery required for PI3K-independent target capture pathway by IpLITR 1.1b.