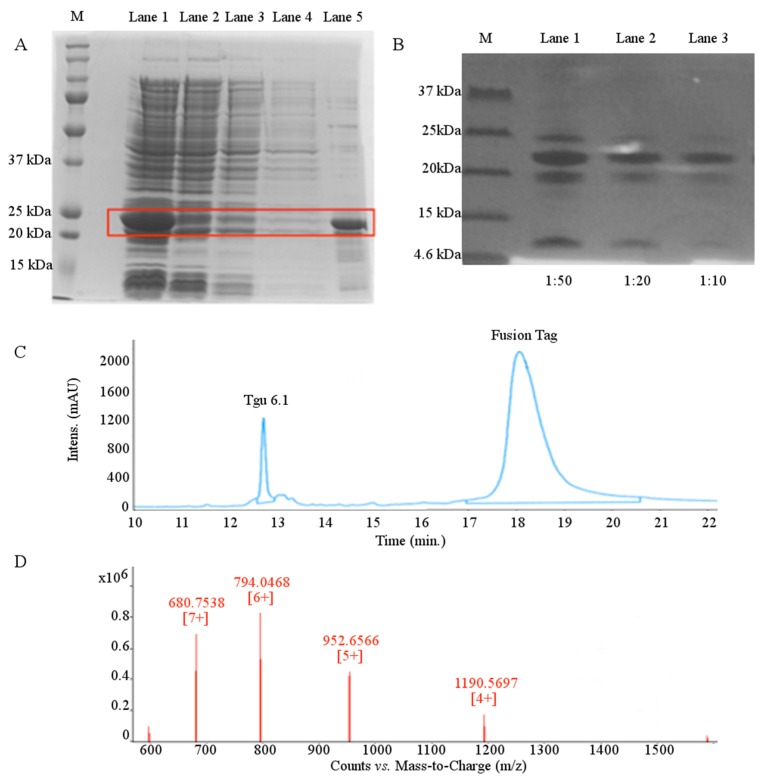
Figure 3.
Expression and purification of Tgu6.1. (A) 12% SDS-PAGE Coomassie-stained gel showing expression and purification of Tgu6.1 fusion protein by Ni-NTA affinity chromatography. M = protein molecular weight marker; Lane 1 = cell lysate; Lane 2 = supernatant post-binding to Ni-NTA resin; Lane 3 = Wash Buffer 1 supernatant; Lane 4 = Wash Buffer 2 supernatant; Lane 5 = imidazole eluted fraction. (B) Tris-tricine 16.5% SDS-PAGE Coomassie-stained gel showing Tgu6.1 cleavage by enterokinase. M = protein molecular weight marker; Lanes 1–3, enterokinase cleavage in 1:50, 1:20 and 1:10 dilutions. (C) Chromatogram of RP-HPLC purification of Tgu6.1 from TRX fusion tag. An X-Bridge C18 semi-preparative column was used with Buffer A (0.1% TFA) and Buffer B (80% ACN/0.1% TFA). The peptide was eluted with a linear gradient of 5%–75% Buffer B over 30 min at a flow rate of 5 mL/min. (D) LC-MS characterization of folded Tgu6.1. The +4, +5, +6 and +7 ion charge states are shown. Expected mass = 4758.58 Da. Observed mass = 4758.28 Da.