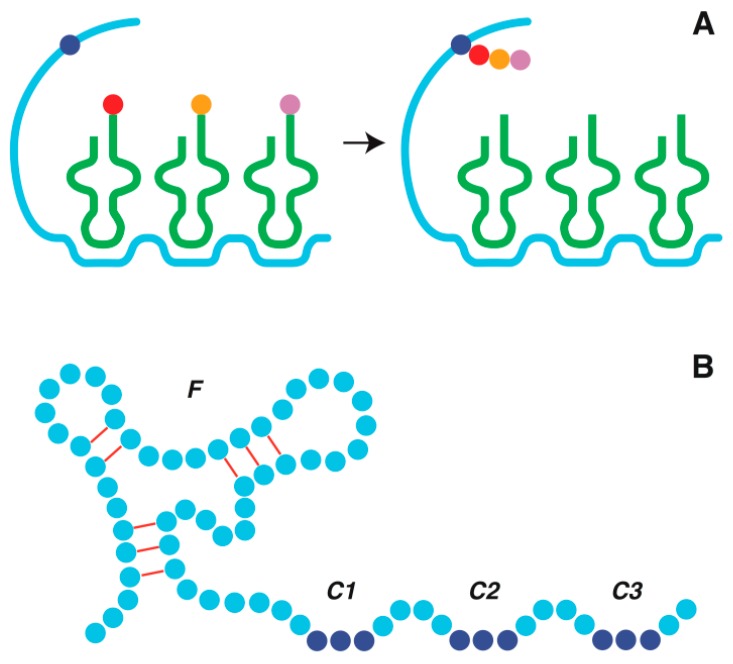
Figure 1.
SART model of primitive messenger RNA. (A) Synthesis of a Leu–Ser–Asp side chain on target nucleotide X (dark blue circle) from Leu–rLeuRS, Ser–rSerRS and Asp–rAspRS conjugates (with Leu represented by purple, Ser by orange and Asp by red circles) bound to amino acid-specific codonic sites on the SART template. Peptide sequence correlates with order of codonic sites on template. Elongation of the peptide may proceed in N → C direction, which entails initial transfer of Leu to Ser–rSerRS, followed by Leu–Ser to Asp–rAspRS, and finally Leu–Ser–Asp to X; or in C → N direction, which entails initial transfer of Asp which is sited closest to X, followed by Ser, and finally Leu from their aa–rARS conjugates to X or growing peptide on X. Both the N → C and C → N modes are workable for short peptides. For long polypeptides, however, the amino acids at the N-terminus that add to the growing peptide last in the C → N mode would be distant from X and the growing peptide on X, rendering their additions problematic. Therefore, the N → C elongation mode was adopted for RNA peptidation, determining thereby also the N → C direction of peptide elongation in modern ribosomal protein synthesis, which differs from RNA peptidation only with respect to its omission of the final transfer of the completed peptide to X. (B) Primitive two-domain fRNA–mRNA as precursor of modern messenger RNA. F, functional aptamer/ribozyme domain; C1–C3, template domain with three triplet codons (dark blue circles).