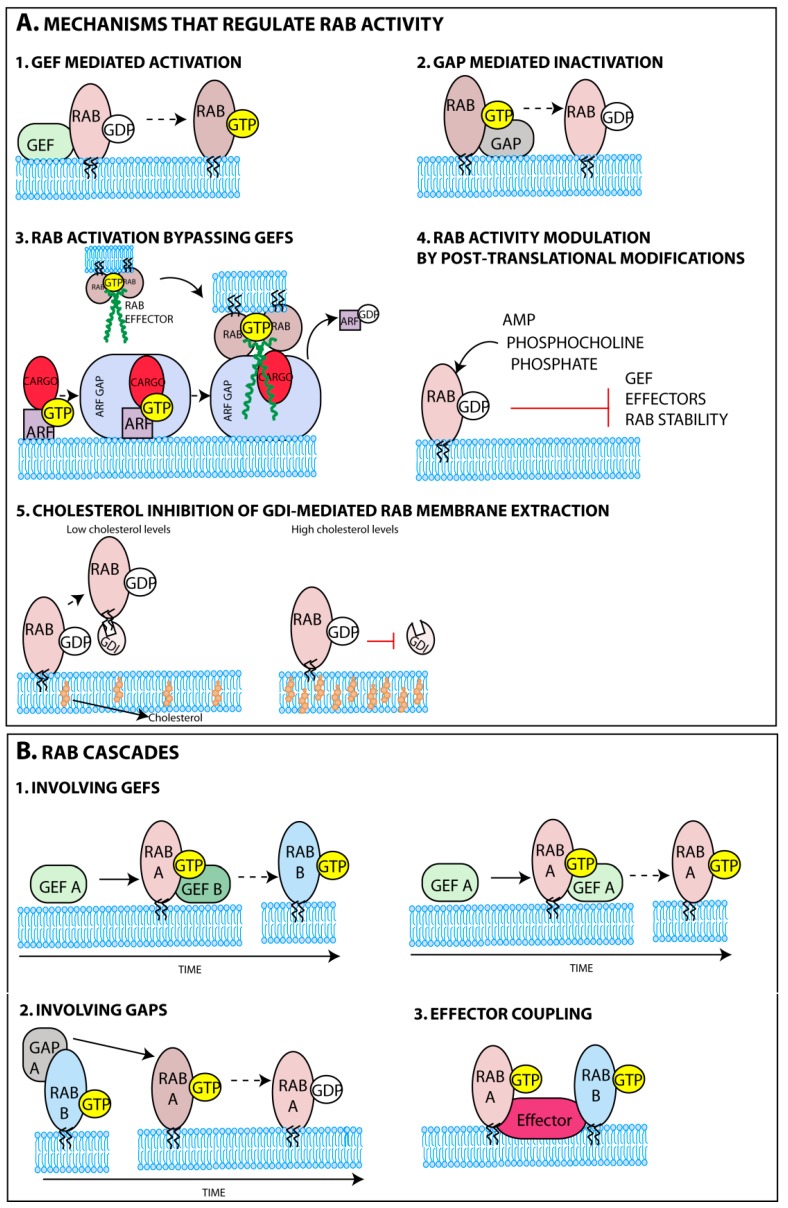
Figure 2.
Rab GTPase spatial and temporal regulation. (A) Activation of Rab GTPases occurs through the exchange of GDP by GTP, a process catalyzed by guanine nucleotide exchange factors (GEFs), with a concomitant conformational change (1). Active Rabs attract multiple effectors and can be converted to the inactive state through GTP hydrolysis (2), which is accelerated by GTPase-activating proteins (GAPs). In certain circumstances, the recruitment of Rabs to membranes can bypass activation by Rab-GEFs. Activated ADP ribosylation factor (ARF) binds its cargo, and the dimer recruits the ARF GAP. The newly formed trimer has also affinity for an effector of a Rab, establishing a crosstalk between a ARF and a Rab (3). Alternatively, post-translational modifications can also regulate Rab function (4). High cholesterol content in membranes can reduce Rab extraction from membranes by inhibiting the activity of the corresponding guanine nucleotide dissociation inhibitory proteins (GDI) (5); (B) Rab GTPase conversion cascades are flexible mechanisms that allow crosstalk between Rabs. The activation (1) or inactivation (2) of a particular Rab can be made by a GEF or GAP, respectively, that was recruited by another Rab that acts upstream. The same Rab can also recruit its own GEF to maintain the activation state by positive feedback loop (1). Distinct Rabs can bind the same effector (3), a process called effector coupling.