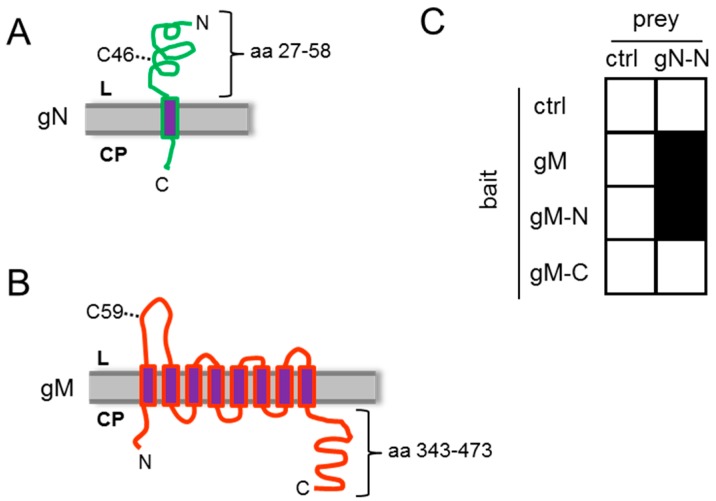
Figure 1.
HSV-1 gN and gM interact via their N-terminal domains. (A) The schematic figure shows the putative localization of HSV-1 gN within cellular membranes. The N-terminal domain faces the luminal (L) side and the C-terminal domain is exposed on the cytoplasmic (CP) side, with a membrane anchor domain near the C terminus (CBS SignalP and TMHMM Server were used for prediction); (B) HSV-1 gM is a membrane protein encoding 8 predicted membrane domains with both N- and C-terminal domains exposed to the cytoplasm; (C) the interacting domains of gM with gN were analyzed by applying the yeast-2-hybrid system. Yeast cells expressing either full-length gM or gM 1–342 (gM–N) fused to Gal4–DBD together with Gal4–AD-coupled N-terminal domain of gN 27–58 (gN–N) were able to grow on medium selective for reporter gene activity, while the cytoplasmic tail of gM 343–473 (gM–C) failed to interact. As a control (ctrl) empty bait and prey plasmids were used.