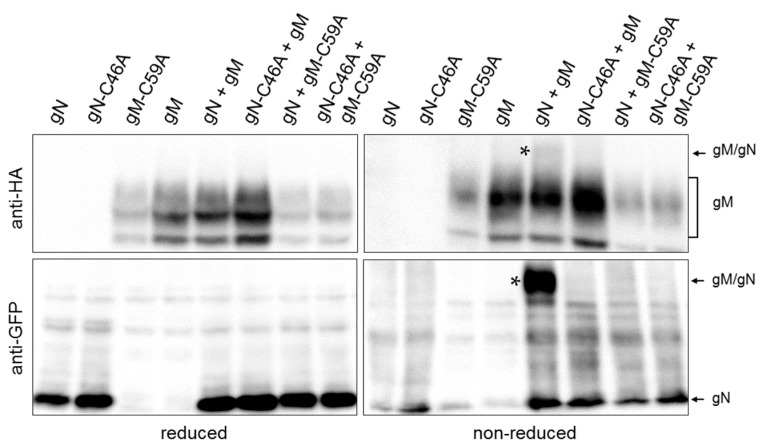
Figure 3.
HSV-1 gN forms a covalent interaction with gM by disulphide bonding between C59 of gM and C46 of gN. To determine whether C59 of gM and C46 of gN form a disulphide bridge, site-directed mutagenesis was performed to replace cysteines (C) by alanines (A). Plasmids encoding gN–EYFP, HA–gM, HA–gM–C59A and gN–C46A–EYFP were transiently transfected into HeLa cells either alone or in combination. Cell extracts were analyzed 24 h.p.t. using reducing and non-reducing SDS-PAGE followed by Western blotting. Proteins were detected using anti-HA and anti-GFP antibodies followed by peroxidase-conjugated secondary antibodies. The complex formed between gN and gM is marked by stars.