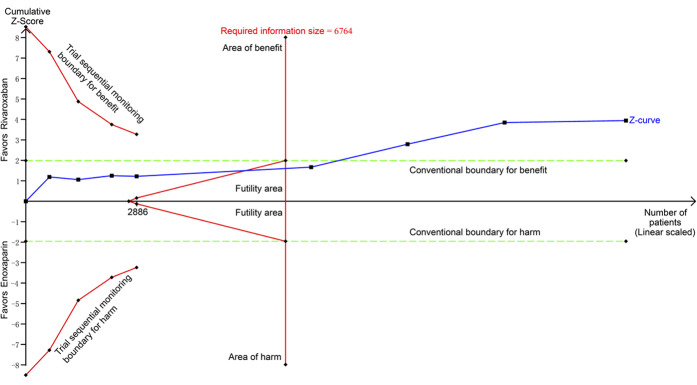
Figure 5. Trial sequential analysis of 9 trials comparing rivaroxaban with enoxaparin for symptomatic venous thromboembolism.
Trial sequential analysis of 9 trials (black square fill icons) illustrating that the required information size had been reached and the cumulative z-curve crossed the traditional boundary, indicating further studies were not needed and were unlikely to change the inferences. A diversity adjusted required information size of 6,764 patients was calculated using α = 0.05 (two sided), β = 0.20 (power 80%), a relative risk reduction of 56.86% based on trials with adequate allocation concealment, and an event proportion of 1.02% in the control arm. X-axis: the number of patients randomized; Y-axis: the cumulative Z-Score; Horizontal green dotted lines: conventional boundaries (upper for benefit, Z-score = 1.96, lower for harm, Z-score = −1.96, two-sided P = 0.05); Sloping red full lines with black square fill icons: trial sequential monitoring boundaries calculated accordingly; Blue full line with black square fill icons: Z-curve; Vertical red full line: required information size calculated accordingly.