FIG 8.
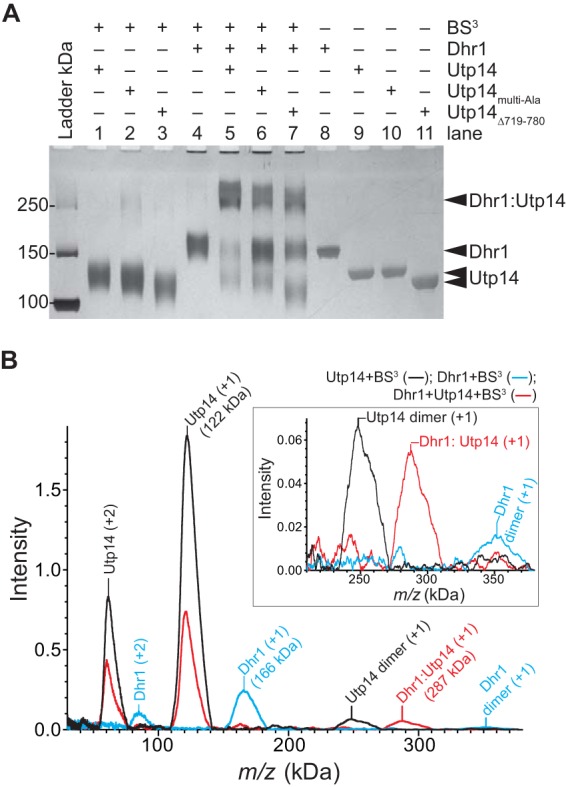
Dhr1 and Utp14 form a 1:1 complex in vitro. (A) Representative cross-linking reactions stopped after 30 min. SDS-PAGE separated the cross-linked protein complex from individual protein. The RT reaction mixture contained a 2 μM concentration of each protein with other reagents described in Materials and Methods. (B) Mass spectrometry analysis of the same samples as in lane 1 (Utp14), lane 4 (Dhr1), and lane 5 (both proteins) in panel A. Masses of the individual proteins, Dhr1 (166 kDa) and Utp14 (122 kDa), have increased by more than 10% due to modification of the large numbers of lysine residues in each protein: 123 and 99, respectively. Upon addition of both proteins, a new mass appears, 287 kDa, consistent with formation of a 1:1 complex. The inset shows an enlargement of the intensity of the masses centered on 300 kDa.
