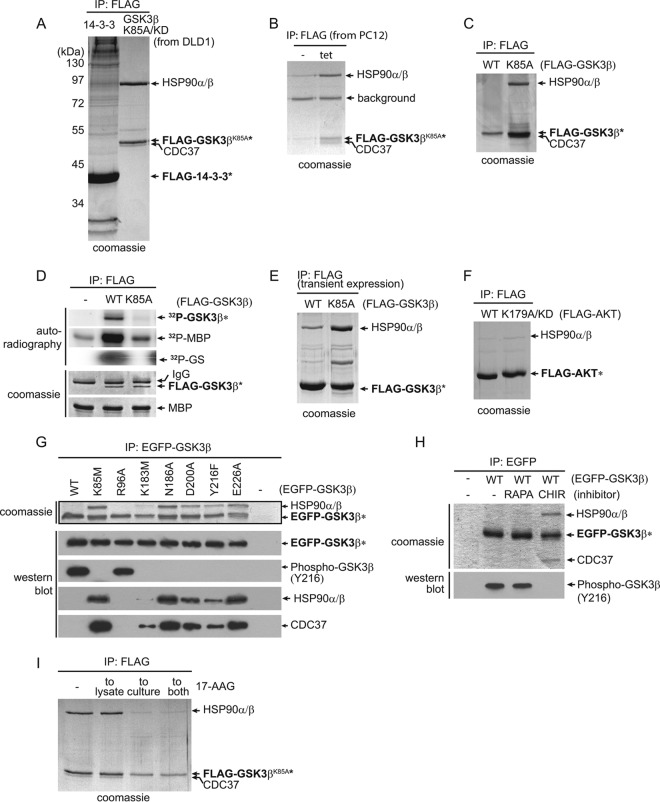
FIG 1.
GSK3β kinase-dead mutants, but not wild-type kinase, form stable complexes with HSP90. (A) Stably expressed FLAG-tagged GSK3βK85A/KD and FLAG-tagged 14-3-3ε were immunoprecipitated from DLD-1 cells, and the immunoprecipitates were resolved by SDS-PAGE. Bait (asterisks) and coprecipitating proteins were visualized by Coomassie staining. Proteins identified by mass spectrometry in the FLAG-tagged GSK3βK85A/KD immunoprecipitate are labeled. (B) GSK3βK85A, stably expressed under a tetracycline (tet)-inducible promoter, in PC12 cells also formed a stoichiometric complex with HSP90 and CDC37. Bands labeled as HSP90 and CDC37 were confirmed by mass spectrometry. (C) Stably expressed wild-type GSK3β (WT) did not bind HSP90 in DLD-1 cells. (D) Kinase assays confirmed the absence of GSK3βK85A activity toward in vitro substrates as measured by 32P-labeled MBP and GS. (E) Under transient-overexpression conditions, GSK3βK85A interacted with HSP90 in DLD-1 cells in an approximately 1:1 ratio, while wild-type GSK3b also bound HSP90 at a reduced but detectable level. (F) FLAG-tagged AKT1WT or KD AKTR179A was stably expressed in DLD-1 cells. Visualization of coprecipitating proteins by Coomassie staining showed no difference between WT and KD mutant AKT1 in binding HSP90. (G) EGFP-tagged GSK3β WT and a series of mutants were created and stably expressed in DLD-1 cells. Following anti-EGFP precipitation, their activities were measured by in vivo autophosphorylation of GSK3β on Y216. Binding of HSP90 and CDC37 was determined by comparing band intensities of Coomassie and Western blot protein bands. Wild-type and GSK3β R96A have similar activities, as measured by Y216 phosphorylation, but do not bind HSP90 or CDC37. (H) DLD-1 cells expressing EGFP-GSK3βWT were either left untreated (−) or treated with mTOR inhibitor rapamycin (RAPA) or GSK3-specific inhibitor CHIR99021 (CHIR). Following anti-EGFP IP of GSK3β and SDS-PAGE, major interacting proteins HSP90 and CDC37 in the CHIR99021-treated cell samples were visualized by Coomassie staining. (I) Treatment of the GSK3bK85A-expressing cells by adding 17-AAG to the culture medium drastically reduced the levels of HSP90 in the GSK3bK85A IP.