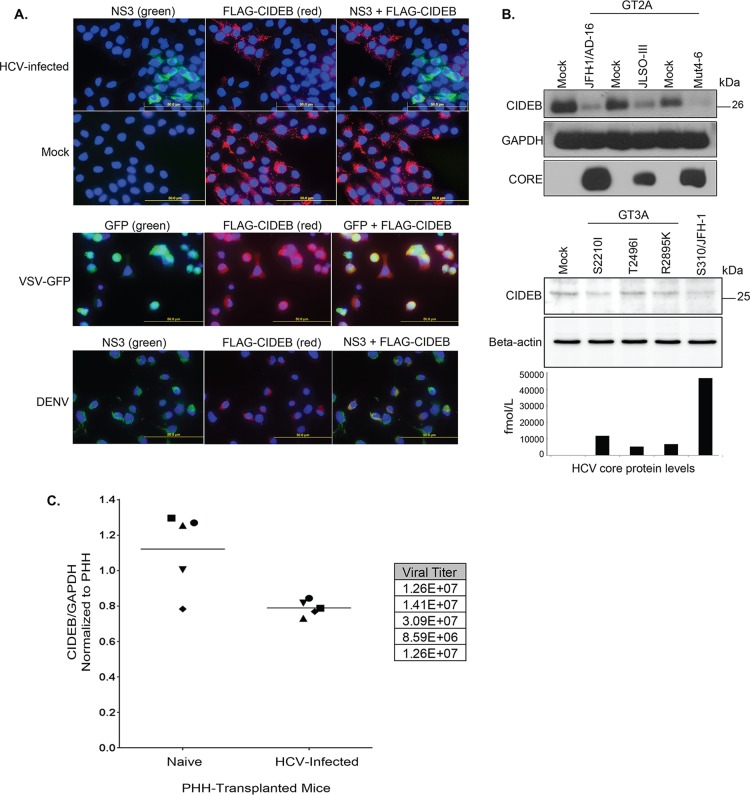
FIG 1.
HCV infection downregulates CIDEB in vitro and in vivo. (A) Immunofluorescence staining of viral proteins and CIDEB. Huh-7.5-based CIDEB-KO cells (clone 3) stably expressing FLAG-CIDEB were infected with a high-titer JFH-1 (HCV) variant, JFH-1/AD16 (genotype 2), for 3 days before costaining for FLAG and HCV NS3. FLAG-CIDEB CBKO#3 cells were infected with VSV-GFP for 16 h, stained for FLAG-CIDEB, and analyzed for coexpression of FLAG-CIDEB and GFP. FLAG-CIDEB CBKO#3 cells were infected with DENV for 48 h and costained for FLAG-CIDEB and DENV NS3. (B) Immunoblot of CIDEB in genotype 2-infected (top) or genotype 3-infected (bottom) cells. Cells were infected for 3 days with various genotype 2a viruses before analysis by Western blotting or for 8 days with various genotype 3a-based (S310) clones or the S310/JFH-1 chimera. On day 8 postinfection, core protein was measured from the supernatants by HCV core-specific ELISA, and cell lysates were analyzed by Western blotting for CIDEB. Beta-actin was included as a loading control. (C) CIDEB protein levels in the HCV-infected uPA/SCID humanized mouse model. Liver tissue lysates from primary human hepatocytes (PHH)-transplanted uPA/SCID mice, either uninfected (n = 5) or HCV infected (n = 5), were subjected to Western blotting to measure CIDEB protein levels. Data from individual mice are plotted as CIDEB protein normalized to GAPDH protein (control). Quantification of band intensity was performed using ImageJ software (National Institutes of Health). HCV-infected versus uninfected humanized mice showed statistically significant levels of CIDEB protein (P = 0.01). Measured HCV titers (in RNA copies per milliliter) are listed in the adjacent table.