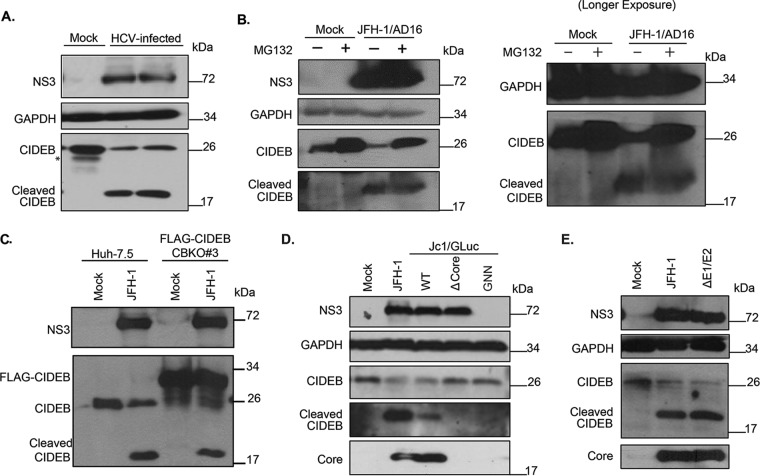
FIG 3.
HCV-infected cells exhibit lower levels of CIDEB protein than do uninfected cells, likely through a proteolytic cleavage event. (A) Effect of HCV infection on endogenous CIDEB protein. Western blot analysis of Huh-7.5 cells infected with JFH-1/AD16 for 3 days revealed a faster-migrating band recognized by the CIDEB antibody, in addition to the full-length product. The asterisk indicates a degradation product occasionally seen from uninfected (mock) Huh-7.5 cells. (B) Effect of proteasome inhibition on CIDEB protein levels in HCV-infected cells. Huh-7.5 cells were infected with JFH-1/AD16 for 48 h and then treated with MG132 for 18 h before analysis by Western blotting. Results obtained with a longer exposure of GAPDH, CIDEB, and the CIDEB cleavage product are shown to the right. (C) Effect of HCV infection on N-terminally FLAG-tagged CIDEB. Shown are the results of Western blot analysis of CIDEB-KO cells (clone number 3) stably expressing FLAG-CIDEB and electroporated with JFH-1 for 3 days. FLAG-CIDEB was detected by anti-CIDEB antibody. (D) Western blot analysis of endogenous CIDEB and NS3 levels in Huh-7.5 cells with various HCV RNAs. Cells were electroporated with 10 μg of RNA generated from either wild-type or mutant genomes and then analyzed 48 h later for the ability to downregulate CIDEB. The Δcore mutant is a Jc1/GLuc variant lacking the intact HCV capsid protein; the GNN mutant is a Jc1/GLuc variant harboring a replication-deficient replicase. (E) Similar analysis of endogenous CIDEB and NS3 levels in Huh-7.5 cells 48 h after cells were electroporated with 10 μg of RNA generated from either wild-type JFH-1 or the ΔE1/E2-JFH-1 mutant, a viral construct lacking the two HCV envelope proteins.