FIG 1.
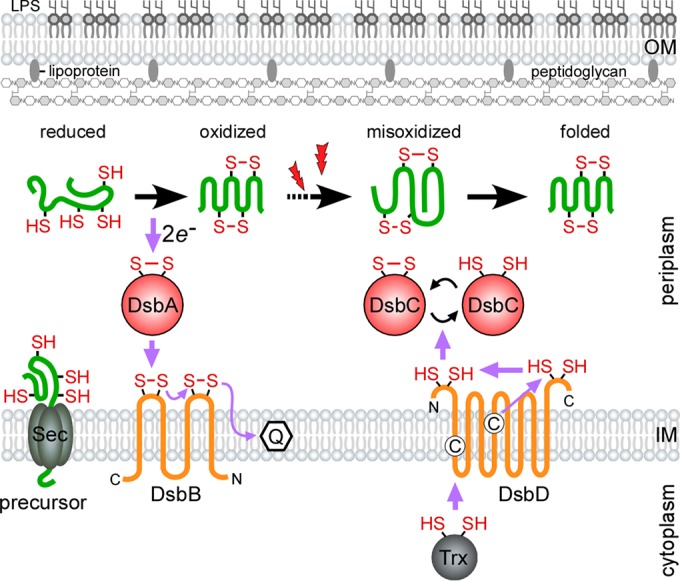
Oxidative protein folding in the Gram-negative periplasm. To catalyze oxidative protein folding, DsbA donates a reactive disulfide bond to reduced protein precursors as they are secreted into the oxidizing periplasm by SecYEG. Following catalysis, the DsbA active site is reoxidized by the transmembrane protein DsbB, which shuttles the gained electrons to the electron transport chain via a conjugated quinone. Extracellular oxidative stress (denoted by lightning bolts) or the lack of DsbA proofreading activity can cause substrates to become misoxidized. Aberrant disulfide bonds are reshuffled by the reductase DsbC. The reducing power of DsbC is maintained by the transmembrane DsbD, which receives electrons from cytoplasmic thioredoxin (adapted from reference 26). The purple arrows denote the direction of the electron flow, and the cysteine residues in the membrane domains of DsbD are shown as circled C's.
