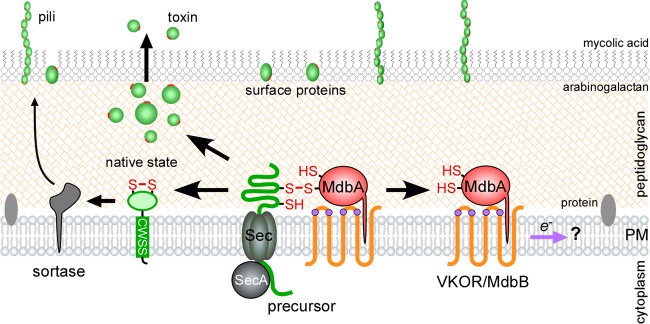
FIG 2.
Oxidative protein folding in the actinobacterial exoplasm. Using pilus proteins and diphtheria toxin as model substrates, oxidative protein-folding pathways have been proposed in the actinobacterial pathogens A. oris and C. diphtheriae. Unfolded pilin precursors are oxidized by the membrane-tethered thiol-disulfide oxidoreductase MdbA. Proper folding is a prerequisite for sortase-mediated assembly of pili on the cell surface. A. oris MdbA is reoxidized by the transmembrane VKOR, while C. diphtheriae MdbA is hypothesized to be recycled by an unidentified factor called MdbB. It is not clear how VKOR or MdbB is reoxidized. The catalytic cysteine residues of VKOR are shown as purple circles, disulfide bonds formed in mature proteins are shown as red lines, and the purple arrow indicates the presumed direction of the electron flow (modified after references 23 and 24).