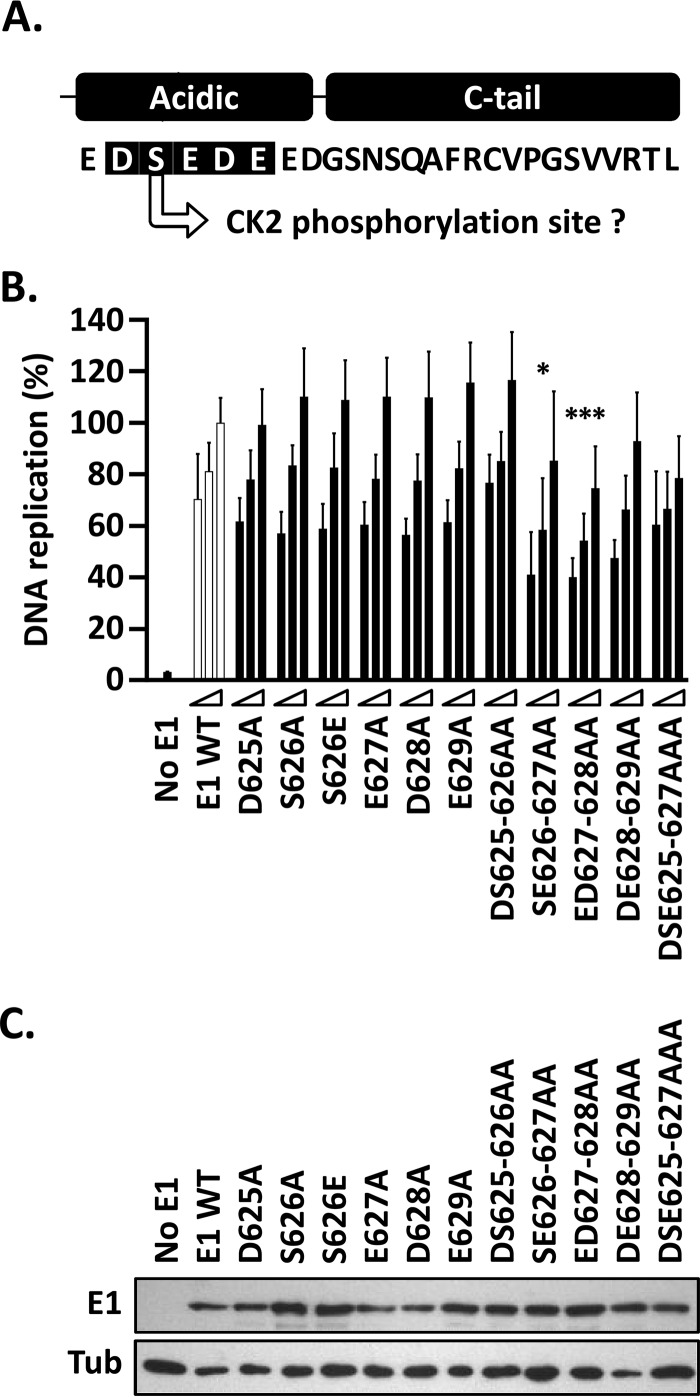
FIG 6.
Single-amino-acid substitutions in the E1 AR have little effect on HPV11 DNA replication. (A) Representation and amino acid sequence of the C terminus of HPV11 E1. The 5 residues within the AR that were subjected to mutagenesis are shaded in black. Also indicated is the fact that serine 626 is a putative CK2 phosphorylation site. (B) Mutant E1 proteins were evaluated for HPV11 DNA replication activity (as for Fig. 3B). Only the mutant proteins that had two or three amino acid substitutions showed a decrease in activity, albeit moderate (10 to 20%). Statistical significance was assessed by comparing the DNA replication activity of each E1 mutant protein to that of wild-type E1 (white bars) using one-way ANOVA followed by Dunnett's post hoc analysis. Significant differences are indicated (*, P ≤ 0.05; ***, P ≤ 0.001). Standard deviations are indicated by the error bars. (C) Expression of GFP-tagged wild-type E1 and mutant derivatives. Extracts from transfected cells were separated on an SDS-12% PAGE gel prior to immunoblotting with an anti-GFP antibody. Tubulin was used as a loading control.