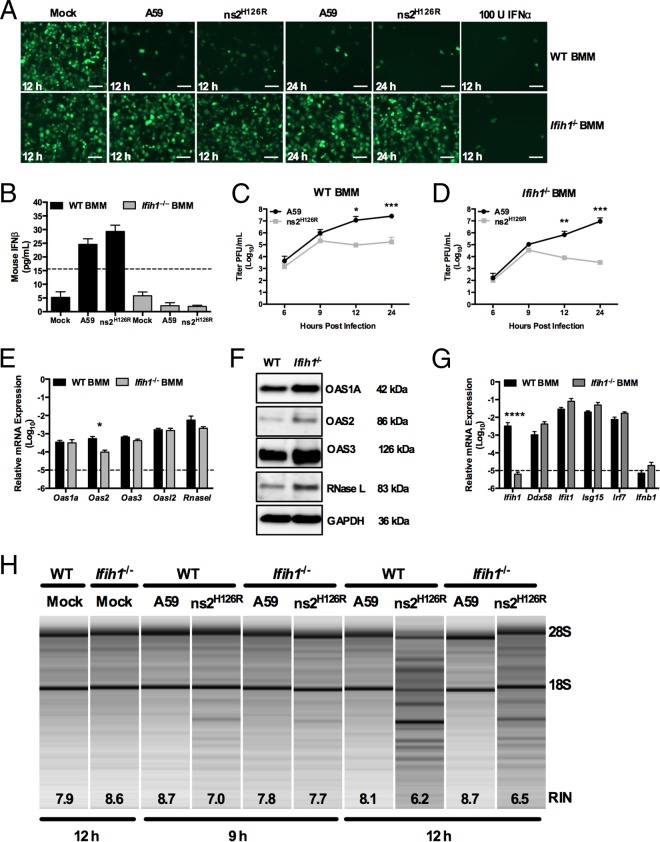
FIG 4.
Infection of Ifih1−/− macrophages with A59 and ns2H126R. (A) WT or Ifih1−/− BMM cultures were either mock infected or infected with A59 or ns2H126R (1 PFU/cell). At 12 h postinfection, the supernatants were treated with UV light to inactivate the virus and incubated with L2 mouse fibroblasts for 24 h, followed by infection with NDV-GFP (1 PFU/cell). As a positive control, L2 cells were treated with IFN-α for 24 h and then infected with NDV-GFP. At 12 or 24 h postinfection, the cells were fixed and examined for EGFP expression by microscopy. The scale bars represent 50 μm. (B) Supernatants taken at 12 h postinfection from the same mock-infected or A59- and ns2H126R-infected WT and Ifih1−/− BMM cultures were analyzed by ELISA for mouse IFN-β with a VeriKine kit. The limit of detection is indicated by the dashed line. The data are from one representative experiment of two. (C and D) WT BMM (C) and Ifih1−/− BMM (D) cultures were infected with A59 and ns2H126R (1 PFU/cell), and at the times indicated, the virus titer was determined by plaque assay of the supernatant. The data are pooled from two independent experiments performed in triplicate and are shown as means ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E and G) mRNA expression levels relative to β-actin mRNA are expressed as 2−ΔCT, where ΔCT is equal to CT Target Gene minus CT β-actin. The dashed lines indicate the lower limit of detection. The data shown are pooled from two independent experiments, each performed in triplicate, and are shown as means and SEM. *, P < 0.05; ****, P < 0.0001. (F) Proteins were extracted from WT and Ifih1−/− BMM and probed by Western blotting with antibodies directed against OAS1A, OAS2, OAS3, RNase L (MAb), and GAPDH. (H) RNA was extracted from infected WT and Ifih1−/− BMM cultures at 9 and 12 h postinfection, as well as cultures 12 h post-mock infection, and rRNA degradation was assessed with a bioanalyzer. 28S and 18S rRNAs are indicated.