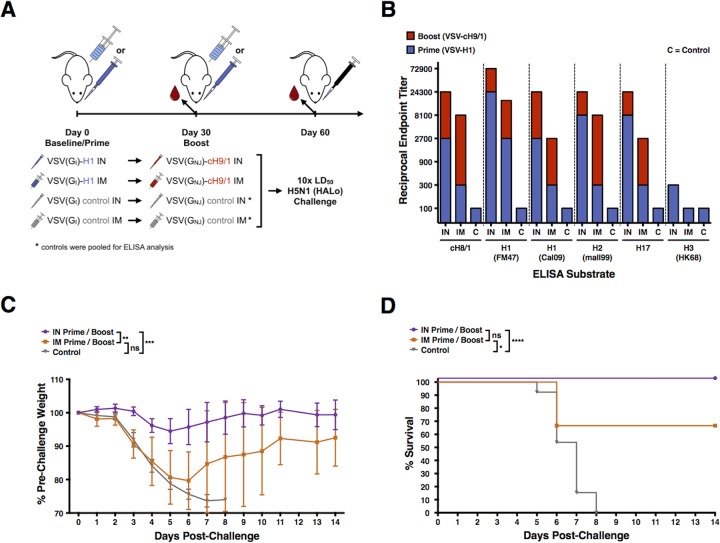
FIG 2.
VSV-H1 and VSV-cH9/1 vaccination and H5N1 challenge experiment. (A) Schematic of the prime-boost-challenge vaccination experiments: i.m. and i.n. vaccinations, H5N1 heterologous influenza challenge. Intranasal vaccinations were performed with 2 × 105 PFU, and i.m. vaccinations were performed with 4.5 × 105 PFU. (B) Serum reciprocal endpoint ELISA titers of mice vaccinated with VSV-H1 (prime) and VSV-cH9/1 (boost). Intramuscular vaccination results in greater boost, but overall serum antibody titers postboost are greater for i.n.-vaccinated animals. (C) Vaccinated mice were challenged i.n. 1 month postboost with 10 LD50 of heterosubtypic H5N1 (HALo) influenza virus. Weights were recorded daily for 14 days postchallenge and are graphically displayed as the mean percentage of the prevaccination weight. Error bars represent the 95% CI of the mean weight. All control mice died or required euthanasia per protocol by day 8 postchallenge. Significant differences between groups are indicated in the graph. (D) Kaplan-Meier survival curves. Significant differences between groups, as identified by a log-rank (Mantel-Cox) test, are indicated in the graph. Two mice from the i.m.-vaccinated group challenged with HALo H5N1 influenza virus died on day 6 postchallenge (66% overall survival). ns, not significant; *, P < 0.05; **, P < 0.005; ***, P < 0.0005; ****, P < 0.0001.