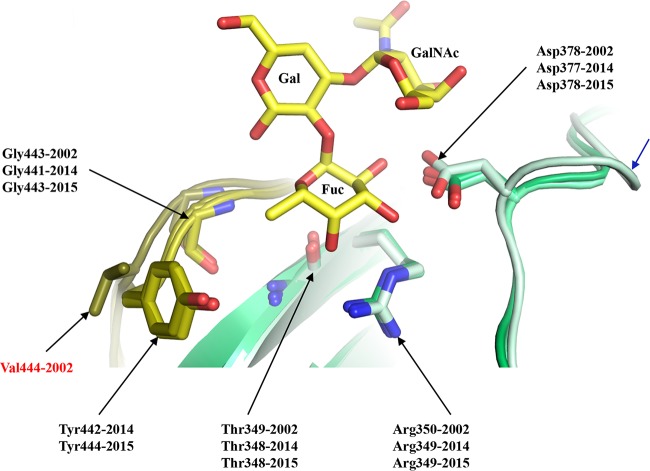
FIG 3.
Model of the GII.17 HBGA binding pocket. The figure presents a superposition of an A-trisaccharide (A-tri; yellow sticks) from the GII.10 P domain A-trisaccharide complex structure (3PA1) onto the GII.17 P domains (colored as described in the Fig. 2 legend), Saitama/T87 (dark olive and dark lime green), Kawasaki323 (olive and lime green), and Kawasaki308 (light olive and light lime green), showing the common set of residues (side chain and main chain) interacting with the fucose (Fuc) moiety of HBGAs. The 2002 Saitama/T87 Val444 residue (labeled in red) was substituted to Tyr442/Tyr444 in 2014 and 2015 (Kawasaki323 and Kawasaki303, respectively). The 2015 Kawasaki308 P domain had an insertion (blue arrow) on the loop hosting Asp378. The A-tri is an α-l-fucose(Fuc)-(1-2)-α-d-galactose(Gal)-(3-1)-N-acetyl-α-d-galactosamine (GalNAc).