FIG 6.
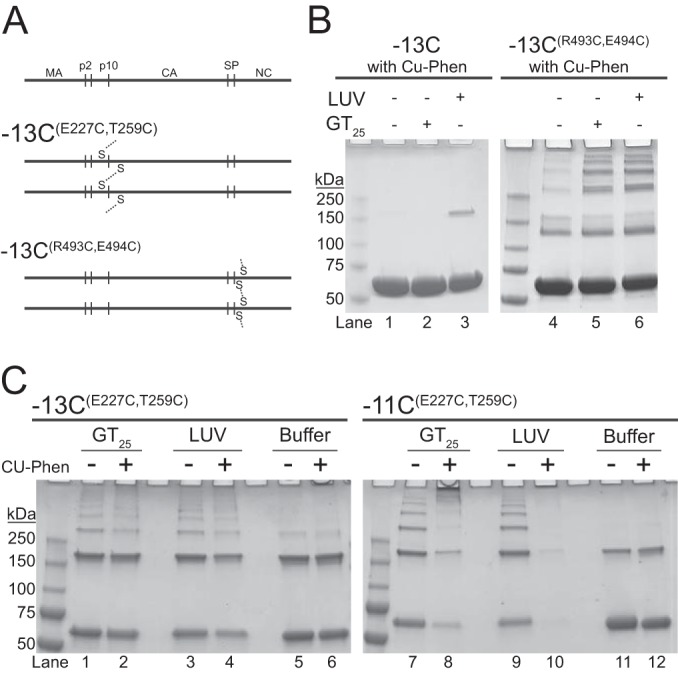
Disulfide cross-linking of membrane-bound Gag. (A) Schematic representation of disulfide bond formation by oxidation. For −13CE227 T259, cross-linking occurs between cysteine-227 and cysteine-259 of a neighboring Gag protein. For −13CR493C E494C, cross-linking occurs between cysteine-493 and cysteine-494 of a neighboring Gag. (B and C) Representative nonreducing Coomassie-stained gels of Gag cross-linking. Monomeric Gag is the first band, corresponding to the midpoint between the 50-kDa and 75-kDa mass marker bands. (B) Cu-Phen induced disulfide cross-linking of the control proteins, −13C (left) and −13CR493C E494C (right). Lanes 1 to 3 and 4 to 6 correspond to reactions with buffer only, plus GT25, and plus LUV, respectively. (C) Cross-linking of −13CE227 T259 (left) and −11CE227 T259 (right). Each condition was tested without (−) and with (+) Cu-Phen under the following conditions; plus GT25, plus LUV, and buffer only. LUVs were composed of POPC/POPS/Chol/PI(4,5)P2 (32/30/36/2).
