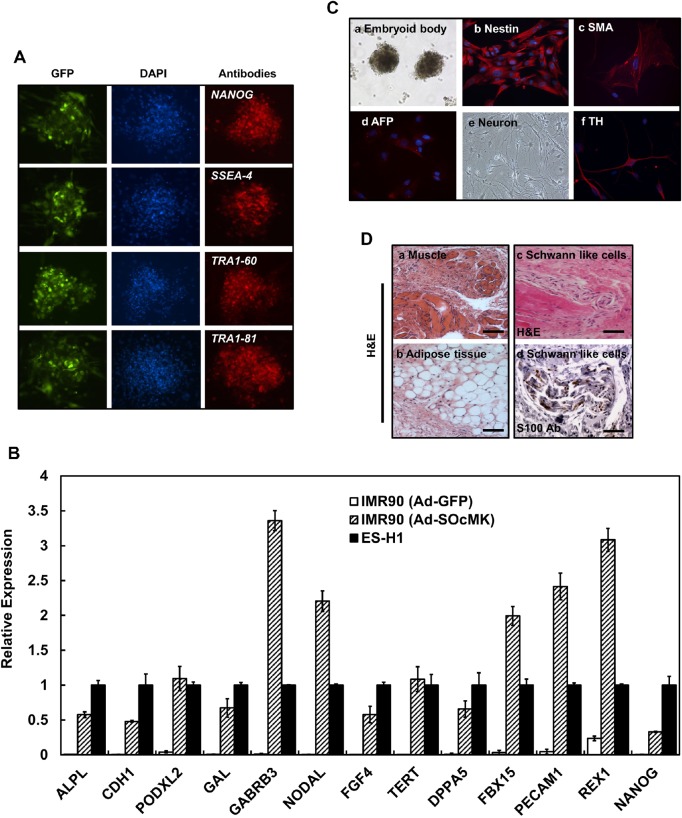
Fig. 3.
Reprogrammed cells with Ad-SOcMK express endogenous ES cell-marker genes and show pluripotency. (A) Reprogrammed cells with Ad-SOcMK were subjected to immunofluorescence study using antibodies against the following: NANOG, SSEA-4, TRA1-60 and TRA1-81. Left panels show expression of GFP, middle panels depict nuclear staining with DAPI. The respective antibody labeling (see Table S5) is shown in the right panels. (B) Expression of ESC marker genes by qPCR is shown. IMR90 cells were transduced with Ad-GFP or Ad-SOcMK. As cells were reprogrammed, total RNA was isolated from harvested cells and subjected to qPCR analyses to determine expression of ES cell-marker genes as indicated in graph. GAPDH RNA was amplified as an internal control. (C) Differentiation of Ad-SOcMK-transduced IMR90 cells. On day 3, Ad-SOcMK-transduced IMR90 cells were mechanically dissociated and cultured in ESC medium (without bFGF) in non-coated T25 flasks. EBs formed after 8-9 days, as observed by phase contrast photomicrograph (a, 4× magnification). Cells in each of the three germ layers were identified with antibodies against the following proteins (see Table S5): Nestin (b) for ectodermal progenitors, SMA (c) for mesodermal progenitors, and AFP (d) for endodermal progenitors. (e,f). After plating on MEF cells, iPSCs differentiated into neuronal cells judged by phase contrast image (e, 10× magnification) and some neurons were stained with dopaminergic marker, tyrosine hydroxylase (TH) (f). (D) Subcutaneous injection of reprogrammed cells resulted in teratoma formation in NOD/SCID mice. Differentiated tissues showing muscle and adipose cell morphology were seen (a,b) as well as Schwann-like cells (H&E stain, scale bars: 100 μm) (c), adjacent section to (d) stained with an antibody against the marker protein S100 (scale bar: 50 μm).