Myelin plays important roles in vertebrates, ensuring the rapid propagation of action potentials and the long-term integrity of axons, but the molecular mechanisms of myelin formation remain poorly understood. Recent studies have demonstrated that myelination is regulated by the TYRO3, AXL (also known as UFO) and MERTK (TAM) family of enzymes, which consists of Tyro3 (also known as Brt, Dtk, Rse, Sky, and Tif), Axl (also known as Ark, Tyro7, and Ufo), and Mer (also known as Eyk, Nym, and Tyro12) along with its ligand growth arrest-specific gene 6 (Gas6) (Binder et al., 2011; Tsiperson et al., 2011; Miyamoto et al., 2015). The three TAMs are also expressed in the brain during postnatal development, specifically, in the white matter, which consists of myelinated axons (Prieto et al., 2000). These studies are helping to identify the signals that are associated with myelination in oligodendrocytes and Schwann cells.
The TAM family members are type I receptor-tyrosine kinases, and each is composed of an Ig-like domain, a fibronectin type 3 (FN3) domain, and a protein tyrosine kinase domain (Godowski et al., 1995). It is well established that TAMs control inflammatory responses, cell proliferation, cell survival, and phagocytosis in cells of various types (Figure 1B). They also mediate extracellular-to-intracellular signal transduction (Godowski et al., 1995). As their endogenous ligands, the TAMs recognize protein S (PROS1) and Gas6, both of which are widely expressed in the central nervous system (CNS) after birth (Prieto et al., 1999). Interestingly, recent works have explained that Gas6 signaling controls oligodendrocyte survival through phosphatidylinositol 3-kinase (PI3-kinase) and upregulates the expression of 2′3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase), which is known as a marker for oligodendrocyte and myelin. It also plays an important role in oligodendrocyte myelination (Shankar et al., 2003). Moreover, Gas6 increased the number of myelinated neurons in an oligodendrocyte progenitor cell (OPC) and dorsal root ganglion (DRG) neuron co-culture (O’Guin et al., 2014). These findings indicate that the TAM-Gas6 signaling pathway promotes the myelination of oligodendrocytes in the nervous system as well as supporting immune responses (Binder et al., 2011). Moreover, Gas6 also promotes the remyelination of oligodendrocytes after cuprizone-induced injury (Tsiperson et al., 2010) and is necessary for oligodendrocyte survival. Taken together, these findings demonstrate that the TAM-Gas6 signaling pathway plays an important role in oligodendrocyte myelination/remyelination and demyelination in CNS. Yet the molecular mechanisms operating within this pathway and underlying the myelin formation process during development remain largely unknown. This review summarizes recent developments in our understanding of the necessity of Gas6-stimulated Tyro3 activation for Schwann cell myelination and/or remyelination.
Figure 1.
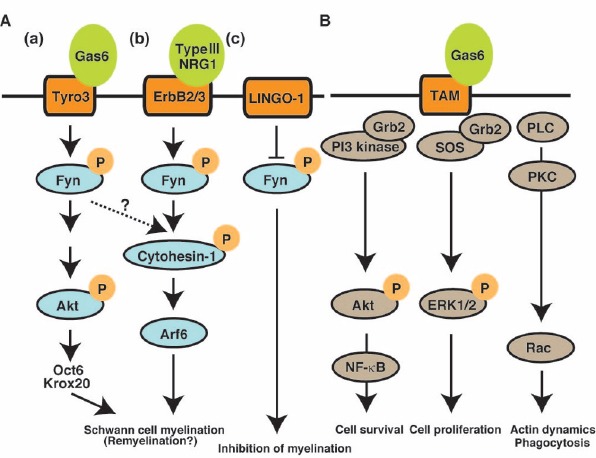
Signaling pathways through Gas6-TAM implicated in Schwann myelination.
(A) Endogenous Gas6, which is secreted by neurons and endothelial cells binds to Tyro3. Since Tyro3 only expresses in Schwann cells, not in oligodendrocytes, as well as no-glial cells, Gas6 induced Tyro3 activation specifically regulates myelination in Schwann cells. Tyro3 downstream molecule Fyn tyrosine kinase is activated by the correlation between axons and Schwann cells. Activated Fyn regulates the expression levels of Oct6 and Krox6, acting through Akt signaling (a). Also, Fyn directly mediates phosphorylation of cytohesin-1, which promotes Arf6 activation (b). Finally, signal transduction in both pathways controls Schwann cell myelination. Gas6/TAM signal transduction participates in cell survival, cell proliferation, and actin rearrangement in oligodendrocytes as well as no-glial cells. (c). LINGO-1 inhibits the phosphorylation of Fyn and oligodendrocytes myelination. (B) Gas6/TAM signaling is well established as regulator of cell survival, cell proliferation, and actin dymanics in non-glial cells. Arf6: ADP-ribosylation factor 6; ERK1/2: extracellular signal-regulated kinase 1/2; Gas6: growth arrest-specific gene 6; Grb2: growth factor receptor-bound protein 2; LINGO-1: leucine rich repeat and immunoglobin-like domain-containing protein 1; NR-κB: nuclear factor kappaB; NRG1: neuregulin 1; Oct6: POU domain, class 3, transcription factor 1; PI3: phosphoinositide-3; PKC: protein kinase C; PLC: phospholipase C; SOS: son of sevenless; TAM: the TYRO3, AXL (also known as UFO) and MERTK; Tyro3: tyrosine-protein kinase receptor.
Gas6-Tyro3 signaling participates in Schwann cell myelination: Previous studies have demonstrated that all of the TAM family members (Tyro3, Axl, and Mer) are expressed in oligodendrocytes and that they are important for oligodendrocyte myelination in the CNS. Interestingly, Tyro3 is the only TAM that is only expressed in Schwann cells. Based on these findings, Miyamoto et al. (2015) analyzed sciatic nerves in Tyro3 knockout mice (Tyro3(-/-)) and demonstrated that loss of Tyro3 causes reduction of myelin thickness in the peripheral nervous system (PNS) compared with wild-type mice as measured at both 3 days and 2 months of age. These results show that the capacity for myelination is significantly reduced in Tyro3(-/-) mice compared with wild-type mice. This in turn suggests that the TAM Tyro3 also regulates the myelination of Schwann cells in the PNS (Miyamoto et al. 2015).
The non-receptor Src-family tyrosine kinase Fyn controls the initial events of myelination in the CNS (Umemori et al., 1994) and regulates cell differentiation, cell polarity, and stability of the cytoskeleton in oligodendrocytes. Fyn is likely activated by NRG1/ErbB2 signaling through axo-glial interaction. It directly binds to cytohesin-1, the activator for the small GTPase Arf6 in the PNS (Figure 1A(b)) (Yamauchi et al., 2012). Arf6 is also known as a regulator of myelination in both the CNS (Akiyama et al., 2015) and the PNS (Yamauchi et al., 2012). NRG1/ErbB2-stimulated Arf6 activation eventually promotes Schwann cell precursor cell migration and myelination in sciatic nerves. Together, these results show that the axon-glia association promotes myelination and activates Fyn in oligodendrocytes and in Schwann cells during development (Yamauchi et al., 2012). On the other hand, a recent study identified Fyn as a new binding partner of Tyro3, mediated by its kinase domain, and it is already known that Tyro3 specifically causes phosphorylation at the 420 tyrosine site of Fyn, which is known to be an activation loop during myelination (Miyamoto et al., 2015). The expression levels of myelin protein zero (MPZ) are remarkably low in Fyn(-/-) Schwann cells. Interestingly, overexpression of active Fyn could impair myelination in Tyro3(-/-) mice. These results indicate that Fyn activation and its downstream signaling molecules are essential for Gas6-Tyro3-induced myelination during development in the PNS. Thus the interaction between Gas6 and Tyro3 similarly regulates myelination and may activate Fyn concurrently with the NRG1/ErbB2 signaling pathway.
Recent works have demonstrated that the PI3-Kinase/Akt/mTOR and ERK1/2-MAPK signaling pathways play important roles in Schwann cell myelination. Also, some studies demonstrated that Gas6-Tyro3 signaling pathway includes phosphorylation of Akt in various cells. On the other hand, the phosphorylation of Akt ((p473) Akt) was significantly decreased in both Tyro3(-/-) and Fyn(-/-) mice after birth (Miyamoto et al., 2015). Since Akt activation is regulated by the phosphorylation of two residues (threonine 307 and serine 473), activated Akt is also necessary for myelination and acts as a downstream molecule of both the Gas6-Tyro3 signaling pathway and the Fyn signaling pathway in Schwann cells. Collectively, Gas6 induced Tyro3 activation directly promote phosphorylation of Fyn and the events play roles myelination, acting through Akt signaling in Schwann cells (Figure 1A(a)).
The expression levels of Krox20 (also known as NAB1/2) and Oct6 (also known as POU3F1) on postnatal day 1 are lower in Tyro3(-/-) mice and Fyn(-/-) mice than in wild-type mice, yet the loss of Tyro3 and that of Fyn do not affect cell proliferation and cell death, respectively, in sciatic nerves. Both transcription factor Krox20 and Oct6 are upregulated before myelination and are involved in controlling Schwann cell myelination. Thus, Gas6-Tyro3 and the downstream Fyn signaling pathway may specifically regulate the expression levels of both Krox20 and Oct6 during Schwann cell differentiation and myelination in the PNS (Figure 1A(a)).
Gas6-stimulated signaling also regulates cell proliferation and cell migration in vascular smooth muscle cells. A previous report has also demonstrated that Gas6 controls Schwann cell proliferation, acting through extracellular signal-regulated kinase (ERK-2) (Li et al., 1996). Similarly, deletion of Tyro3 resulted in inhibition of Gas6-stimulated proliferation and migration in Schwann cells. Thus, Gas6-stimulated TAM activation is required for cell proliferation and cell migration in Schwann cells as well as in non-glial cells.
These studies may contribute to the discovery of future therapies for patients with Charcot-Marie-Tooth (CMT) disease, an inherited peripheral neuropathy associated with decreased myelin thickness and peripheral nerve injury. Continued investigation of the unresolved questions regarding the TAM family, including Tyro3, may enable us to identify targets for the therapeutic manipulation of myelination in disease states.
Prospective clinical treatment for myelin regeneration: Patients with CMT and CMT-related disorders exhibit progressive degeneration of the peripheral nerves. A growing body of studies has identified more than 60 genes that are involved in causing these diseases, and the molecular pathogenesis has been described (Bouhy et al., 2013). Based on these data, many CMT animal models have been generated. In particular, patients with CMT1A, which is caused by the duplication of a 22-kDa peripheral myelin protein (PMP22), exhibit peripheral neuropathy with demyelination. To date, researchers have focused on identifying drugs that can induce myelination and/or remyelination using these CMT model mice. It has been demonstrated that treatment with neurotrophin-3 (NT3) promotes remyelination in the sciatic nerves of CMT1A model mice. It has also been suggested as a potential clinical treatment for CMT and other diseases that cause demyelination.
As stated above, recent studies have shown that Gas6-Tyro3 signaling is necessary for Schwann cell myelination (Miyamoto et al., 2015). These findings indicate the likelihood that controlling Gas6-Tyro3 signaling may enable a new therapy for CMT that would improve quality of life for CMT patients. A study has also demonstrated that Gas6-stimulated TAM (Tyro3, Axl, and Mer) activation suppresses demyelination and significantly promotes remyelination in the spinal cord (Gruber et al., 2014). In addition, the signal transduction protects axons in the CNS during experimental autoimmune encephalomyelitis (EAE) (Gruber et al., 2014). Although the expression levels of Gas6 mRNA spontaneously increased in the sciatic nerves after axotomy, clinical treatment with the TAM-specific agonists Gas6 and/or PROS1, both of which induce Tyro3 activation, may promote PNS remyelination in CMT model mice or myelin regeneration after PNS nerve injury. Also, Gas6 treatment may accelerate myelination and/or differentiation in the sciatic nerves after transplantation of induced pluripotent cells (iPS cells) or other stem cells into the lesions. If this is confirmed, Tyro3 will represent a promising target for the clinical treatment of demyelination in patients with CMT or peripheral nerve injury.
Based on accumulating evidence that ERK1/2 signaling engages in cross-talk with the Fyn downstream signaling pathway and with transmembrane receptor tyrosine kinase (RTK) signal transduction including Tyro3 signaling in myelination (Gonsalvez et al.. 2015), the ERK1/2 signaling pathway may promote remyelination after PNS nerve injury and/or demyelination in patients with CMT1A. Also, these treatments may delay the progression of certain CMT phenotypes. Drug, which is target for these proteins, may be one of options for clinical treatment, after the Gas6-TAM signaling pathwaythrough MEK1/2-ERK signaling pathway will be characterized well.
On the other hands, leucine rich repeat and immunoglobin-like domain-containing protein 1 (LINGO-1) is known as negative regulator for oligodendrocytes myelination via inhibition of phosphorylation of Fyn. It is also well known that blocking of LINGO-1 using anti-LINGO-1 antagonist antibody promotes oligodendrocytes differentiation, myelination, and remyelination (Zhang et al., 2015). Based on these findings, amyloid precursor protein (APP), interacts with LINGO-1, blockage may enhance myelination through LINGO-1, which could be of a crosstalk with TAM signaling. These LINGO-1 antagonists may be available for clinical treatment of demyelination.
Finally, we have established a new signaling pathway in which Gas6-Tyro3 signaling, acting through Fyn and Akt, involves and may act in concert with NRG1/ErbB2 signaling to affect Schwann cell myelination and remyelination (Figure 1A). This evidence may be expected to help develop clinical treatments that will promote remyelination and regeneration in sciatic nerves.
This work was supported by Grants-in-Aid from the Japanese MEXT and MHLW. This work was also supported by the Innovative Areas’ Scientific Research (Glial Assembly) and the Takeda Science Foundation.
References
- Binder MD, Cate HS, Prieto AL, Kemper D, Butzkueven H, Gresle MM, Cipriani T, Jokubaitis VG, Carmeliet P, Kilpatrick TJ. Gas6 deficiency increases oligodendrocyte loss and microglial activation in response to cuprizone-induced demyelination. J Neurosci. 2008;28:5195–5206. doi: 10.1523/JNEUROSCI.1180-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godowski PJ, Mark MR, Chen J, Sadick MD, Raab H, Hammonds RG. Reevaluation of the roles of protein S and Gas6 as ligands for the receptor tyrosine kinase Rse/Tyro 3. Cell. 1995;82:355–358. doi: 10.1016/0092-8674(95)90424-7. [DOI] [PubMed] [Google Scholar]
- Gonsalvez D, Ferner AH, Peckham H, Murray SS, Xiao J. The roles of extracellular related-kinases 1 and 2 signaling in CNS myelination. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.04.024. doi: 10.1016/j.neuropharm.2015.04.024. [DOI] [PubMed] [Google Scholar]
- Gruber RC, Ray AK, Johndrow CT, Guzik H, Burek D, de Frutos PG, Shafit-Zagardo B. Targeted GAS6 delivery to the CNS protects axons from damage during experimental autoimmune encephalomyelitis. J Neurosci. 2014;34:16320–16335. doi: 10.1523/JNEUROSCI.2449-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Guin KN, Gruber RC, Raine CS, Guzik HM, Poulos BK, Shafit-Zagardo B. Gas6 enhances axonal ensheathment by MBP+ membranes processes in human DRG/OL promyelinating co-cultures. ASN Neuro. 2014;6:e00135. doi: 10.1042/AN20130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto AL, Weber JL, Lai C. Expression of the receptor protein-tyrosine kinases Tyro-3, Axl, and mer in the developing rat central nervous system. J Comp Neurol. 2000;425:295–314. [PubMed] [Google Scholar]
- Shankar SL, O’Guin KN, Cammer M, McMorris FA, Stitt TN, Basch RS, Varnum B, Shafit-Zagardo B. The growth arrest-specific gene product Gas6 promotes the survival of human oligodendrocytes via a phosphatesitol 3-kinase-dependent pathway. J Neurosci. 2003;23:4208–4218. doi: 10.1523/JNEUROSCI.23-10-04208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiperson V, Li X, Schwartz GJ, Raine CS, Shafit-Zagardo B. Gas6 enhances repair following cuprizone-induced demyelinateion. PLoS One. 2010;5:e15748. doi: 10.1371/journal.pone.0015748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Torii T, Takada S, Ohno N, Saitoh Y, Nakamura K, Ito A, Ogata T, Tanoue A, Yamauchi J. Involvement of the Tyro3 receptor and its intracellular partner Fyn signaling in Schwann cell myelination. Mol Biol Cell. 2015;26:3489–3503. doi: 10.1091/mbc.E14-05-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Initial events of myelination involve Fyn tyrosine kinase signaling. Nature. 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi J, Miyamoto Y, Torii T, Takashima S, Kondo K, Kawahara K, Nemoto N, Chan JR, Tsujimoto G, Tanoue A. Phosphorylation of cytohesin-1 by Fyn is required for initiation of myelination and the extent of myelination during development. Sci Signal. 2012;5:ra69. doi: 10.1126/scisignal.2002802. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang YP, Pepinsky B, Huang G, Shields LBE, Shields CB, Mi S. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp Neurol. 2015;266:68–73. doi: 10.1016/j.expneurol.2015.02.006. [DOI] [PubMed] [Google Scholar]


