Abstract
Purpose
We quantified fundus autofluorescence (FAF) in the nonhuman primate retina as a function of age and diets lacking lutein and zeaxanthin (L/Z) and omega-3 fatty acids.
Methods
Quantitative FAF was measured in a cross-sectional study of rhesus macaques fed a standard diet across the lifespan, and in aged rhesus macaques fed lifelong diets lacking L/Z and providing either adequate or deficient levels of omega-3 fatty acids. Macular FAF images were segmented into multiple regions of interest, and mean gray values for each region were calculated using ImageJ. The resulting FAF values were compared across ages within the standard diet animals, and among diet groups and regions.
Results
Fundus autofluorescence increased with age in the standard diet animals, and was highest in the perifovea. Monkeys fed L/Z-free diets with either adequate or deficient omega-3 fatty acids had significantly higher FAF overall than age-matched standard diet monkeys. Examined by region, those with adequate omega-3 fatty acids had higher FAF in the fovea and superior regions, while monkeys fed the diet lacking L/Z and omega-3 fatty acids had higher FAF in all regions.
Conclusions
Diets devoid of L/Z resulted in increased retinal autofluorescence, with the highest values in animals also lacking omega-3 fatty acids. The increase was equivalent to a 12- to 20-year acceleration in lipofuscin accumulation compared to animals fed a standard diet. Together these data add support for the role of these nutrients as important factors in lipofuscin accumulation, retinal aging, and progression of macular disease.
Keywords: autofluorescence, lutein, zeaxanthin, omega-3 fatty acids, lipofuscin
Fundus autofluorescence (FAF) is an inherent property of the retina that originates primarily from lipofuscin in RPE cells. Autofluorescent components of lipofuscin accumulate as a result of the formation of bisretinoid fluorophores in photoreceptor outer segments as a byproduct of the visual cycle, followed by their phagocytosis by the RPE.1,2 Increased lipofuscin accumulation in photoreceptors and/or RPE cells can cause a reduction in cellular function and has been associated with aging and retinal disease, including age-related macular degeneration.3–7 Excessive lipofuscin autofluorescence also is a key pathologic feature in several monogenic retinal degenerative conditions. The most notable of these are recessive Stargardt disease and other syndromes caused by mutations in the gene for ABCA4, the flippase that removes all-trans-retinal from the interior of outer segment disk membranes and, therefore, modulates its availability.8–11 Conversely, lipofuscin accumulation is greatly diminished by vitamin A deficiency and by genetic conditions, such as RPE65 mutations, that block the normal retinoid cycle.12–15 Loss of RPE cells and photoreceptors, as in geographic atrophy, results in reduction or abolition of FAF. Due to the autofluorescent properties of lipofuscin and the clinical implications of its accumulation in the eye, quantitative FAF (qFAF) has been explored as a methodology to examine patterns of lipofuscin accumulation in humans with and without retinal disease, to aid in diagnosis and to assess disease progression and retinal aging.5,8,10,11,16–22
Under normal circumstances, the retina is offered some protection from oxidative and light exposure stresses through the accumulation of lutein (L) and zeaxanthin (Z). Lutein/zeaxanthin are xanthophylls (dihydroxy carotenoids) that selectively accumulate in the human and nonhuman primate fovea and are responsible for its characteristic yellow color. These yellow plant-based pigments are concentrated in the central fovea at levels as high as 1 mM to form the macular pigment.23 Lutein/zeaxanthin serve two well-established complementary roles within the retina: first, as antioxidants protecting against free radical damage and second, as filters for high-energy short-wavelength (blue) light to protect the fovea from photooxidation. More recent studies also have demonstrated powerful anti-inflammatory effects and modulation of the immune system, including reduction in complement activation.24,25 All of these processes have key roles in the pathogenesis of age-related macular degeneration (AMD).
Another dietary factor, the omega-3 fatty acids (ω-3′s), including docosahexaenoic acid (DHA) and its precursor eicosapentaenoic acid (EPA), have multiple roles contributing to retinal health.26,27 Docosahexaenoic acid is a major constituent of photoreceptor and neural membranes and is critical for normal retinal function.28–31 It counters inflammation by blocking conversion of the n-6 fatty acid, arachidonic acid, to a series of inflammatory eicosanoids and by activating anti-inflammatory nuclear hormone receptors.26 Docosahexaenoic acid also is the precursor of the docosanoids, including protectins and resolvins.32 In vitro, neuroprotectin D1 (NPD1) is synthesized by RPE cells in response to oxidative challenge and serves to protect these cells from oxidative stress-induced apoptotic cell death. Bazan et al.32 proposed that reductions in NPD1 may contribute to the breakdown of RPE cell homeostasis and the resulting retinal changes seen in aging and retinal diseases. Interactions between L/Z and ω-3 fatty acids may enhance their joint effects. For example, higher DHA intake has been shown to increase the uptake of L/Z and/or its deposition in the fovea.33,34
Several studies have shown that higher levels of L/Z in the diet, blood, or retina are associated with a lower prevalence or slower progression of AMD,35–37 and a reduced risk of progression to advanced AMD was confirmed in a recent randomized clinical trial of L/Z supplementation.38 Similarly, a significantly lower risk of advanced AMD has been found in people with higher dietary intakes of DHA and/or EPA, or higher intakes of fish, their primary dietary source.39–43 However, few studies have directly examined whether severely restricted levels of L/Z and ω-3′s result in accelerated retinal aging and/or retinal disease. Such studies have been lacking because human studies cannot provide strict lifelong control of dietary intakes and other environmental variables, whereas lifelong experimental dietary restriction in animal models generally is too expensive and time-consuming.
In a previous study from our laboratory, monkeys fed lifelong diets free of L/Z, and with either adequate or deficient levels of ω-3′s, had no L/Z present in serum and no detectable macular pigment.44 In that study, monkeys fed the L/Z-free diet, regardless of their ω-3 intake, had altered RPE cell density profiles compared to monkeys fed a standard diet containing adequate levels of L/Z and ω-3′s.45 They also showed increased vulnerability to coherent blue light damage within the fovea, while those also deficient in ω-3′s had increased damage in the parafovea.46 The present study used groups of monkeys fed similar long-term diets, using qFAF as a measure of lipofuscin accumulation to further characterize diet-induced changes in retinal health and susceptibility to accelerated retinal aging or disease. In addition, changes were examined across the lifespan in monkeys fed standard diets providing sufficient quantities of both classes of nutrients.
Methods
Animals and Diets
All experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center and were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
A cross-sectional set of qFAF images was acquired from 67 control rhesus monkeys (Macaca mulatta) ranging from 3 to 26 years of age that were fed a standard laboratory diet (Lab Diet Monkey Diet 5037/5038) providing adequate levels of L/Z and ω-3′s and supplemented with fresh fruits and vegetables. In addition, qFAF images were acquired from rhesus macaques (ages 18–24 years, n = 17) fed one of three L/Z-free semipurified diets from birth. All semipurified diets had the same overall level of fat as the standard diet. The three diets were identical except for their sources of dietary fat, which were designed to provide widely different content of ω-3′s: (1) A high α-linolenic acid (ALA; 18:3ω-3) diet (ω-3 adequate; n = 7), with soybean oil as the sole dietary fat, contained 8% (as weight % of total fatty acids) of ALA, the precursor of DHA, and 54% linoleic acid (18:2ω-6), the primary dietary omega-6 fatty acid providing a ω-6:ω-3 ratio of 7. This diet has been shown to support normal levels of retinal DHA. (2) The second diet (also ω-3 adequate; n = 3) consisted of a mixture of plant, animal, and fish oils that provided the preformed long-chain polyunsaturated fatty acids: 0.6% DHA (22:ω-3), 0.2% EPA (20:5ω-3), and 0.2% arachidonic acid (20:4ω-6); plus 1.4% ALA. This diet led to retina levels of DHA similar to the high ALA group, although circulating levels of DHA were higher. In our previous studies of retinal function,28,47 results were equivalent between the latter two diet groups, and, therefore, data for the two groups were combined in this study to increase n. (3) The ω-3–deficient diet (n = 7) contained <0.3% ALA and 54% linoleic acid, with an ω-6:ω-3 ratio of 180, from a 1:1 mixture of safflower and peanut oils. In addition to the low level of ALA, the high ω-6:ω-3 ratio reduces ALA uptake and DHA synthesis due to competition between the two fatty acid classes. We previously showed that this diet, fed during gestation and from infancy, reduced retina levels of DHA by 80% and altered retinal function.28,29,47
Image Collection and Analysis
Monkeys were anesthetized by an intramuscular injection of Telazol (1:1 mixture of tiletamine hydrochloride and zolazepam hydrochloride, 3.5–5 mg/kg) and maintained with ketamine (1–2 mg/kg) as required, or by inhalant isofluorane (1%–2%) vaporized in oxygen. Supplemental oxygen was provided as needed via nasal cannula at 0.5 to 1.0 L/min, and heart rate and peripheral blood oxygen saturation were monitored by pulse oximetry. Rectal temperature was maintained between 37.0°C and 38.0°C by water-circulating heated pads placed underneath the animal. Animals were positioned prone with the head supported by a chinrest. Before image acquisition, the pupils were dilated to a minimum of 8 mm using phenylephrine (2.5%) and tropicamide (1%) eye drops. Speculums were used to keep the eyelids open, and clear plano contact lenses were inserted centered over the cornea. Following imaging, the contacts and speculums were removed and erythromycin ointment was applied to each eye. Animals then were recovered from sedation and returned to their home cages or enclosures.
A Heidelberg Spectralis SD-OCT system with a standard 30° lens was used to acquire quantitative FAF images from both eyes. We developed a modified method for quantification of FAF based on those reported previously.18 Before acquiring qFAF images, the retina was visualized and brought into focus using the infrared imaging mode. The system then was switched to its short-wavelength excitation laser (488 nm), and final focus adjustments were made to optimize the visualization of the retina. The fundus was first bleached with a minimum 30-second exposure to the blue laser to minimize contribution from photoreceptors to the FAF signal.18 The nonnormalized acquisition setting was used with a manual sensitivity set to 90 for each image. The signal/noise ratio was enhanced by averaging 100 scans. Although an internal reference was not used, the same settings were used for all animals, and all images were acquired by the same operator within an approximately 3-month time period, thus minimizing variation in laser power.
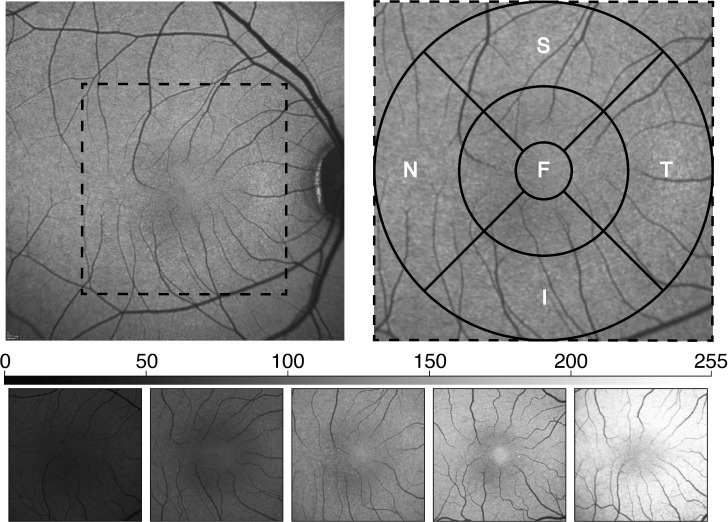
For analysis of gray value (MGV), the acquired FAF image for each animal was imported into ImageJ and a customized macro was used to crop the image into a 6-mm square centered on the fovea. Using a customized Region of Interest Manager macro, the cropped image then was segmented into 9 regions of interest (ROIs): a 1-mm diameter circle centered on the fovea, and 1- to 3-mm and 3- to 6-mm diameter annuli divided into superior (S), nasal (N), temporal (T), and inferior (I) quadrants (Fig. 1). Hereafter, the 1-mm diameter foveal region will be referred to as the foveal center, the 1- to 3-mm annulus as the parafovea, and the 3- to 6-mm annulus as the perifovea. To calculate the mean FAF for each ROI, ImageJ converted each pixel of each RGB image to grayscale, and then divided the sum of the gray value of each pixel within a particular ROI by the total number of pixels for that ROI. The qFAF values of all 9 ROIs were analyzed simultaneously. One eye of each animal was used in the analysis; all eyes were free of opacities.
Figure 1.
Methodology of image segmenting and representative images covering the range of MGV observed. Upper left: The acquired image, obtained from the average of 100 nonnormalized scans, is cropped to a 6-mm square centered on the fovea (dotted square). Upper right: The cropped images then were segmented into 9 ROIs by first centering 1 mm (fovea), 3 mm (parafovea), and 6 mm (perifovea) diameter circles on the foveal center. The para- and perifoveal annuli then were further divided into S, N, T, and I quadrants. Bottom: FAF images from animals representing the range of observed MGV values (∼50 to ∼230) averaged over the total segmented area.
Statistical differences among diet groups were assessed by a linear mixed model to determine main effects of diet and region and their interaction, with post hoc pair-wise comparisons for differences between diets, using IBM SPSS version 22 (released 2013, IBM SPSS Statistics for Windows, Version 22.0; IBM Corp., Armonk, NY, USA). Regional differences within each group then were evaluated by a linear fixed effects model with Bonferonni pairwise analyses as appropriate. In the standard diet animals, the linear fixed effects model included age as a covariate. P values were considered significant at an α level <0.05.
Results
ROI Comparisons
For standard diet animals, when average qFAF values were analyzed for all 9 ROIs, the fovea had lower values than the S and N parafovea (P = 0.023 and P < 0.001, respectively) and all perifoveal regions P < 0.001), whereas the I parafovea was lower (P = 0.001), and T parafovea did not differ from the fovea (Fig. 2, top). In addition, each quadrant of the perifovea was significantly brighter than the corresponding quadrant of the parafovea (all P < 0.001). Within the parafoveal region, FAF was lower in the I quadrant than in all other sectors (I vs. T, P = 0.013; I vs. S and N, P < 0.001), and also was lower in the T quadrant than in either the S or N quadrants (P = 0.015 and 0.001, respectively). In the perifovea, the I quadrant again had lower FAF intensity than the other three quadrants (all P < 0.001). To better illustrate these regional differences while normalizing for overall qFAF differences among animals, we determined the relative FAF of each region by calculating the ratio of the regional MGV divided by the average MGV of all 9 regions (Fig. 2, bottom).
Figure 2.
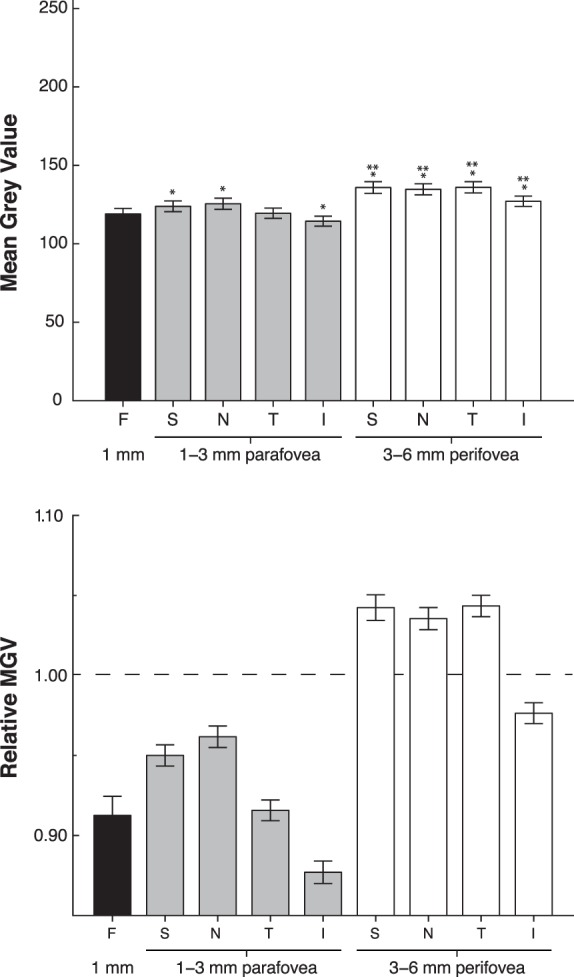
Mean gray values derived from FAF images of standard diet control animals (mean ± SEM, n = 67). *Statistical significance between ROI and fovea. **Statistical significance between the para- and perifoveal annuli within the same quadrant. Top: When divided into all 9 ROIs, the S, N, and I regions of the parafovea were brighter than the fovea, and all four perifoveal quadrants were brighter than the fovea. In addition, all four perifoveal quadrants were significantly brighter than the corresponding parafoveal region. Lower: Relative MGV illustrating the regional differences in FAF. For calculation of relative MGV, the weighted average of each ROI was divided by the average MGV of the total area. The dotted line represents the average FAF of the total segmented area.
Effects of Diet
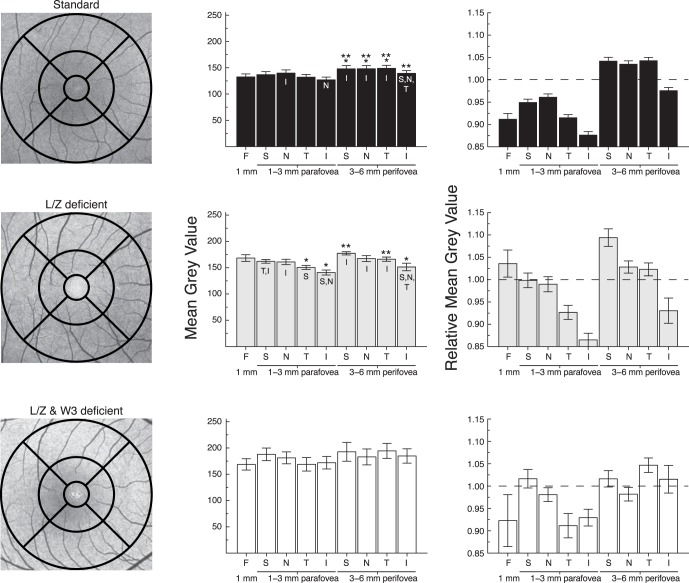
For the L/Z-deficient groups, the average FAF value for each ROI was compared to a subset of age-matched standard diet controls (n = 27; mean age, 20.1 years; range, 15−26 years). A linear mixed model analysis showed a significant main effect of diet, main effect of region, and diet by region interaction (all P < 0.001). In pairwise comparisons among the three diet groups, the two L/Z-free groups did not differ from each other, but both differed significantly from the age-matched standard diet group (P = 0.016 for the adequate ω-3 group and P = 0.001 for the ω-3–deficient group).
Group differences also were examined by region. In the foveal 1 mm center, both the L/Z-free/adequate ω-3 group (P = 0.003) and L/Z-free/ω-3–deficient group (P = 0.01) had higher FAF than the standard diet animals (Fig. 3). This trend continued across all regions for both groups; however, the L/Z-free/adequate ω-3 group was significantly brighter than the standard diet group only in the S parafovea (P = 0.04) and the S perifovea (P = 0.035), and not in the other regions. In contrast, the group deficient in both L/Z and ω-3 had significantly more intense FAF than the standard diet animals in all 9 ROIs (all P < 0.01 except N perifovea, P = 0.022). They did not differ significantly from the L/Z-free/adequate ω-3 group in any region (all P > 0.05).
Figure 3.
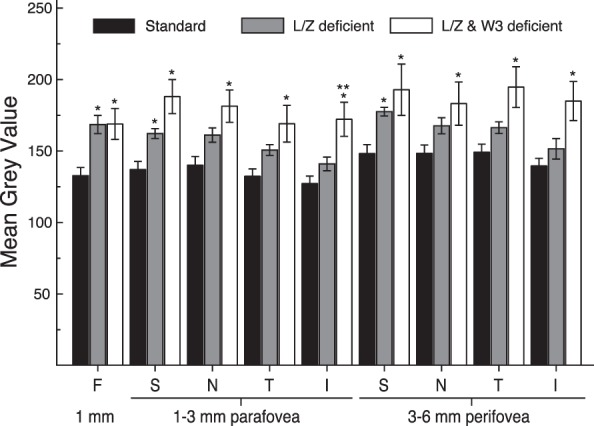
Mean gray values (mean ± SEM) measured in all 9 ROIs of age-matched standard diet fed monkeys (n = 27, black bars), L/Z-deficient/ω-3 FA–adequate diets (gray bars), and L/Z- and ω-3–deficient diet (white bars). *Significant difference between the deficient diet groups and the standard diet group within each respective ROI. **Significant difference between the two deficient diets. The L/Z-deficient/ω-3 FA–adequate diet animals were significantly brighter than standard diet in the fovea and the superior region of the para- and perifovea. The L/Z- and ω-3–deficient diet monkeys were brighter than the standard diet animals in all regions of the para- and perifovea. The L/Z-deficient and the L/Z- and ω-3–deficient diet monkeys were only statistically different from each other in the inferior parafovea.
The regional qFAF patterns within the L/Z-free groups also differed from those in the standard diet group (Fig. 4). Unlike the standard diet animals, the L/Z-free/adequate ω-3 group did not have reduced FAF in the fovea relative to para- or perifoveal sectors, as expected due to their absence of macular pigment. Indeed, their foveal FAF values were higher than in the T and I parafovea (both P < 0.01) and I perifovea (P = 0.048). Within the parafovea, their FAF values were lower in the I than in the S or N regions (P ≤ 0.001), and lower in T than in S (P = 0.04). In the perifovea, the I region was again lower than all other quadrants (P < 0.001 versus S and N, P = 0.015 versus T). For the L/Z-free/ω-3–deficient animals, no differences among regions were statistically significant.
Figure 4.
Regional differences observed in FAF of age-matched standard, L/Z-deficient/ω-3 FA–adequate, and L/Z- and ω-3–deficient diet groups. Left column contains representative FAF images from each diet group corresponding to their average MGV. Central pigmentary disturbances are due to drusen, which are found commonly in aged monkeys. Middle column illustrates raw MGV for each diet group, and right column illustrates the corresponding relative MGV (mean ± SEM). The dotted lines on the relative MGV graphs represent the average MGV across all ROIs. *Statistical significance between the ROI and the fovea. **Statistical significance between parafoveal and perifoveal regions within each quadrant. Letters within the bars indicate significant differences among quadrants within the parafoveal or perifoveal annuli. In standard diet monkeys, regional differences were evident, with the perifoveal region being brightest. A similar pattern was observed in the L/Z-deficient/ω-3 FA–adequate group with the exception of a relatively bright fovea, likely due to minimal signal attenuation from the lack of macular pigment. Finally, although the L/Z- and ω-3–deficient diet animals' values were significantly higher overall, they did not show regional differences in FAF.
Changes With Age
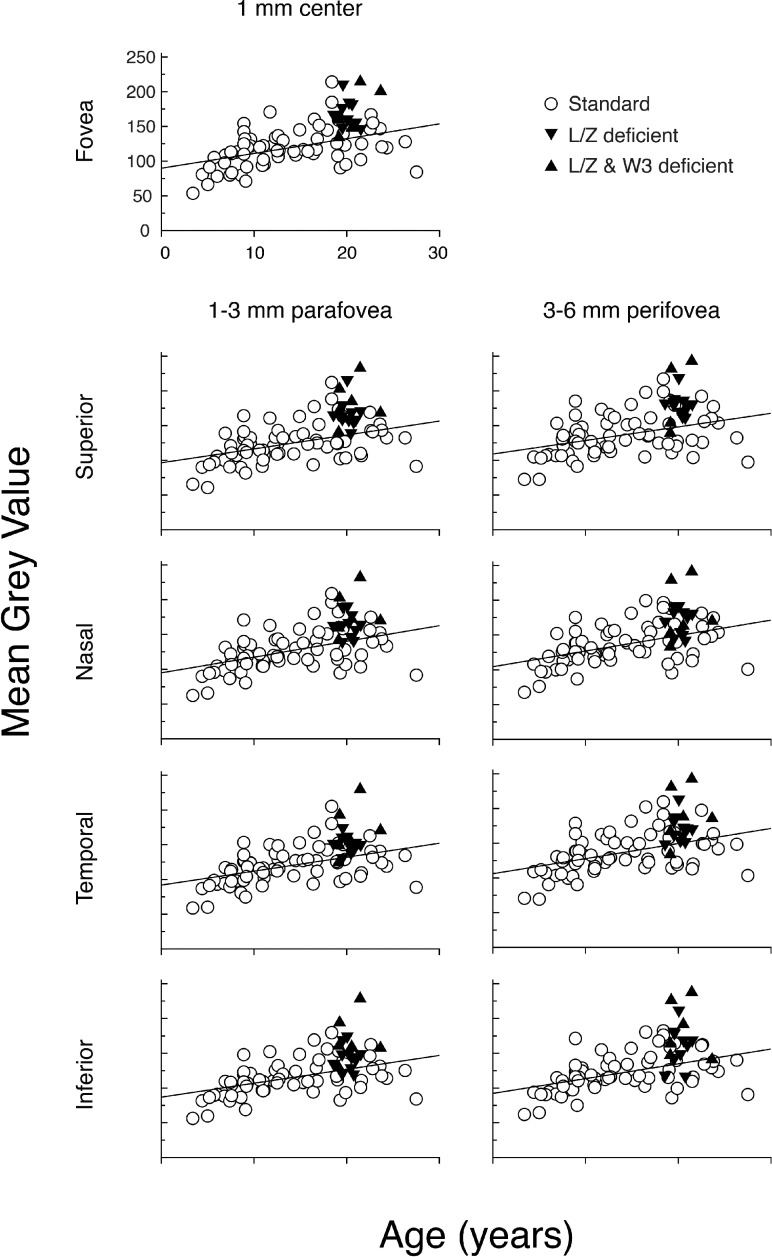
To determine the relationship between FAF and age, we performed a cross-sectional analysis on 67 standard diet monkeys from 3 to 26 years of age. The FAF values for each of the 9 ROIs were plotted as a function of age and the slope was estimated by linear regression (Fig. 5). In all 9 ROIs, FAF values increased with age, and the slopes were similar across all regions (see Table). Figure 5 also plots the FAF values for the 17 L/Z-free animals, with mean ages of 20.1 years for the L/Z-free/adequate ω-3 group and 20.5 years for the L/Z-free/ω-3–deficient group. In all 9 ROIs, most of the L/Z-free animals had FAF values above the linear regression line of the age-matched standard diet animals.
Figure 5.
Scatterplots of MGV for all standard diet control animals (n = 67) for each of the 9 ROIs (open circles) compared to L/Z-deficient (downward solid triangles) and L/Z- and ω-3–deficient monkeys (upward solid triangles). The solid line in each panel represents the best-fit linear regression for the standard diet group. There was significant variability within the standard diet fed control group, with MGV values ranging from 50 to 230. There was a clear trend toward an increase in MGV with age in all regions. Most animals fed deficient diets had MGVs on or above the linear regression line. Axis labels in the Fovea panel are applicable for all panels.
Table.
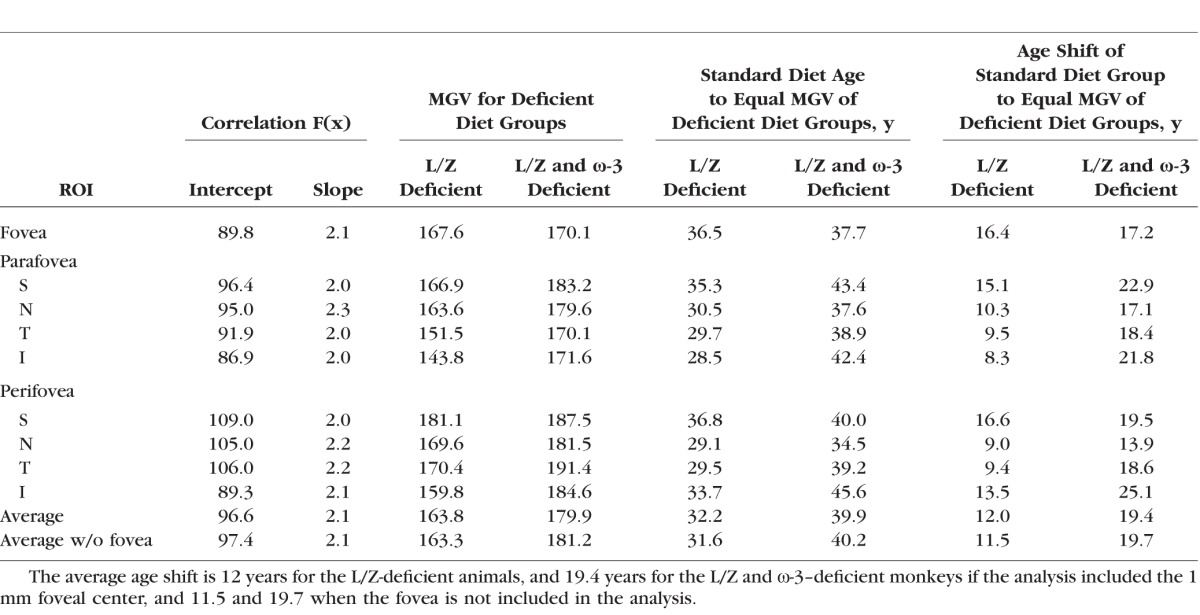
Estimation of the Acceleration of the Age-Related Increase Autofluorescence in the Deficient Groups Compared to Monkeys Fed the Standard Diet, Based on Comparison of the Regression Functions for MGV as a Function of Age
Discussion
This study quantified FAF as a measure of retinal health and aging in nonhuman primates over the lifespan, and as affected by lifelong dietary deficiencies of the macular pigments L/Z and ω-3 fatty acids. In monkeys fed a standard, nutritionally complete laboratory diet supplying adequate L/Z and ω-3 fatty acids, FAF increased progressively with age. The highest FAF levels were found in the perifovea, while conversely the fovea had lower autofluorescence than the superior and nasal parafovea and all regions of the perifovea. In addition, in the para- and perifovea, FAF was lower in the inferior quadrant than in all other sectors; in the parafovea, the temporal quadrant also had lower FAF intensity than the superior and nasal quadrants. The increase with age and the spatial distribution of FAF, including higher levels in the perifovea and lower levels in the fovea and inferior quadrant, replicated the findings of several human studies as determined by both in vivo FAF21,48,49 and tissue measurements of lipofuscin.50 Since lipofuscin components originate in photoreceptor outer segments, their accumulation may vary with photoreceptor density. In human and macaque retinas, the highest density of rods is found in the perifovea and peaks in the superior retina, in parallel with FAF values,51,52 and in human retina, FAF correlates with rod density.53
In animals fed lifelong diets devoid of L/Z, levels of FAF were significantly higher overall than in age-matched animals fed a standard diet containing L/Z. In the subgroup consuming adequate dietary ω-3 fatty acids, FAF was significantly higher in the fovea and superior para- and perifovea than in the standard diet group, whereas monkeys deficient in both L/Z and ω-3 fatty acids had significantly higher FAF than the standard diet group in all nine regions. Unlike in animals fed standard diets, the excitation light was not attenuated by macular pigment in the central foveal region in L/Z-free animals, with a resulting increase in fluorescence emission. This result confirms the common assumption that the foveal dip in FAF seen in normal human and nonhuman primate eyes is in part due to screening of the excitation light by macular pigment. However, in the normal human retina, lipofuscin is lower in the fovea than in the perifoveal ring even when measured in vivo by autofluorescence using excitation wavelengths outside the absorption spectrum of macular pigment,49 or by fluorometric measurements of retinal tissue;50 therefore, lower foveal FAF is not entirely due to macular pigment screening but also other factors, such as higher foveal RPE melanin.54 The foveal dip also may be related to the protection provided to the fovea by macular pigment, through reduction of blue light exposure and antioxidant mechanisms. Thus, the lack of a foveal dip in L/Z-free animals may indicate a true increase in lipofuscin accumulation compared to animals fed normal diets, in addition to lack of macular pigment screening. This will need to be confirmed by postmortem biochemical measurements. However, the L/Z-free/ω-3–deficient animals had significantly higher FAF than age-matched controls in all regions, including those that do not have optically measurable levels of macular pigment. Thus, while lack of macular pigment screening may partially explain why the L/Z-free/ω-3–deficient animals have brighter FAF in the fovea, it would not account for the group differences in FAF in the para- and perifovea.
In our previous study of monkeys reared and maintained on similar diets, the area occupied by autofluorescent lipofuscin granules was measured in the central 6 mm along the superior and inferior meridians, and was increased in those on the L/Z-free/ω-3–deficient diet, particularly in the parafovea at 0.69 and 1.22-mm eccentricity (Leung I, et al. IOVS 2006;47:ARVO E-Abstract 288). Although these animals were younger (8–19 years old) than the current groups, these data provide a general confirmation and morphologic correlate to the current in vivo imaging results.
Lutein and zeaxanthin are concentrated most highly in the central fovea, with highest levels in Henle's fiber layer (cone axons) where they are best positioned to serve as a blue light filter. However, they also are present throughout the retina in photoreceptor outer segments and at lower levels in the RPE.55,56 Indeed, it has been estimated that L/Z in outer segments accounts for 10% to 25% of the total amount in the retina. Levels are higher in the perifovea than in peripheral retina.56 These levels outside the central fovea produce negligible blue light filtering but can act as highly efficient scavengers of reactive oxygen species, thus reducing oxidative damage. Although oxidized lipids are not a source of FAF based on their different fluorescence spectra,57 oxidative damage and lipofuscin accumulation appear to be linked in other ways. Dietary deficiencies of antioxidants, including vitamin E, have been shown to increase lipofuscin accumulation,58–62 as has treatment with prooxidants, such as iron.63 Conversely, in RPE cells in vitro, the addition of vitamin E or L/Z decreases lipofuscin formation.64,65 Bhosale et al.66 showed in peripheral human RPE that levels of L/Z were inversely correlated across subjects with levels of N-retinylidine-N-retinylethanolamine (A2E), a major bisretinoid fluorophore. Furthermore, in a quail model, they showed that dietary supplementation with either L or Z prevented the age-related increase in retinal A2E concentrations seen in unsupplemented birds. This study also showed that the spatial distribution of A2E in the human retina, measured by high performance liquid chromatography (HPLC) and mass spectroscopy, differed from the distribution of FAF; A2E was highest in the periphery, whereas FAF peaked centrally, with highest levels in the perifovea. However, A2E is only one of the bisretinoids contributing to lipofuscin autofluorescence, and similar protective effects of L/Z also may apply to these other components. For example, A2-PE, the immediate precursor of A2E that forms in the photoreceptor outer segments, in some situations may have higher fluorescent intensity than the A2E formed in the RPE.67
In addition, in the RPE, bisretinoids act as photosensitizers and produce a series of potentially toxic oxidation products when exposed to blue light.61,68 Both the blue light filtering and antioxidant properties of L/Z may reduce production of these photooxidation products. Kim et al.69 showed that L and Z robustly blocked the blue light–induced photooxidation of A2-PE. This protective effect of L/Z may be a mechanism for reducing photoreceptor and RPE damage, but its relationship to FAF intensity is complex. In some cases (e.g., oxidation of double bonds on the long arm of the A2E molecule) the blue light-induced fluorescence of oxidized bisretinoids is less than the parent bisretinoids, so that their accumulation would be expected to reduce, rather than enhance, lipofuscin autofluorescence. However, other oxidative modifications (e.g., on the short arm of A2E), have been shown to increase fluorescence several-fold, and, thus, have been proposed as a source of enhanced FAF.67
With respect to ω-3 fatty acids, there are fewer studies examining specific links to lipofuscin accumulation. Doernstauder et al.70 found that DHA supplementation reduced retinal A2E levels and delayed age-related loss of retinal function in wild type mice and in mutant ELOVL4 transgenic mice, a model of dominant Stargardt disease that shows excessive A2E buildup followed by retinal degeneration. The mechanism for such an effect is unknown. Although DHA is highly oxidizable, it and its docosanoid derivatives show powerful protective effects against RPE oxidative stress.26,71 This and other factors also may contribute to increased FAF in animals deficient in L/Z or ω-3 fatty acids to the extent that these deficiencies impair general photoreceptor health and function. For example, reduction in the efficiency of clearance of all-trans-retinal within photoreceptors, or impairment of the process of photoreceptor disk shedding and RPE phagocytosis, can lead to buildup of bisretinoids in outer segments.
The results of this study strongly support the hypothesis that nutritional deficiencies in macular carotenoids and ω-3 fatty acids have a critical role in premature retinal aging and may contribute to the risk of AMD. To estimate the degree of diet-induced acceleration of FAF accumulation with age, we extrapolated the regression line for the standard diet animals to the FAF levels seen in the two deficient groups, which had a mean age of 20 years, equivalent to 60 years in humans given the commonly accepted macaque:human age ratio of 1:3. To reach the average FAF level of the L/Z-free/ω-3 adequate group, the standard diet animals would need to age to approximately 32 years, equivalent to 96 human years, and to reach the FAF of the L/Z-free/ω-3–deficient group they would need to age to approximately 40 years, or 120 human years. Thus, these estimates represent an age shift of approximately 12 and 20 years, respectively, or 36 and 60 years in human terms. A summary of these calculations is provided in the Table.
Multiple previous studies have demonstrated the ability to quantify FAF in human populations. A significant challenge for interpreting data collected clinically by multiple different groups using various devices prompted a recent study to incorporate an internal fluorescence reference that was then used to compare FAF measured using multiple methods.18 The purpose of that approach was to create a standardized method to enable comparison among different clinical imaging systems, equipment operators, and clinical study sites. While it is important to maintain consistency in such a context, our approach did not include an internal reference because our study used the same device, operator, sensitivity setting (gain), and protocol for the collection of all qFAF images from our nonhuman primates, and images were collected over a relatively short time frame (less than 3 months), reducing concern regarding laser power drift. Thus, while we cannot compare our FAF values to those obtained clinically with other confocal scanning laser ophthalmoscopes, the present study was designed specifically to study differences between groups of monkeys under tightly controlled conditions. In addition, in the present study, we did not account for the potential attenuation of FAF due to opacities in the lens or ocular media; however, none of the analyzed eyes had visible opacities. If the deficient groups did have an increase in lens yellowing or more subtle opacities, these could have reduced 488 excitation intensity and, to a lesser extent FAF emission,49 despite age-matching of the standard diet group; the result of such a difference in these animals would be an underestimation of the level of FAF and the differences between the diet groups.
To our knowledge, our study has provided the first detailed information in nonhuman primates on the impact on lipofuscin accumulation of two primary factors in accelerated lipofuscin accumulation, nutrition and aging, which are well described for their involvement in AMD. It is noteworthy that the typical American diet is low in xanthophylls and ω-3 fatty acids, so that increased intake of these nutrients has the potential to improve retinal health and delay the advance of age-related retinal disease. Thus, determining how long-term diet deficiencies contribute to the onset of AMD, and how lipofuscin is related to other pathologic processes, deserve significant further attention. Nonhuman primates on controlled diets provide a particularly valuable resource for exploring these issues.
Acknowledgments
The authors thank Francois Delori, PhD, for editing the manuscript and for helpful advice; and Alexandra Bemis and the staff of the Department of Comparative Medicine at the Oregon National Primate Research Center for their valuable technical assistance and support.
Supported by National Institutes of Health (NIH; Bethesda, MD, USA) Grants R01EY13199, R01DK29930, and P51OD011092, and grants from the Foundation Fighting Blindness and from Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the University of Illinois.
Disclosure: T.J. McGill, None, L.M. Renner, None; M. Neuringer, None
References
- 1. Sparrow JR,, Kim SR,, Wu Y. Experimental approaches to the study of A2E, a bisretinoid lipofuscin chromophore of retinal pigment epithelium. Methods Mol Biol. 2010; 652: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sparrow JR,, Kim SR,, Cuervo AM,, Bandhyopadhyayand U. A2E, a pigment of RPE lipofuscin, is generated from the precursor, A2PE by a lysosomal enzyme activity. Adv Exp Med Biol. 2008; 613: 393–398. [DOI] [PubMed] [Google Scholar]
- 3. Sparrow JR,, Nakanishi K,, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000; 41: 1981–1989. [PubMed] [Google Scholar]
- 4. Sparrow JR. Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration. Adv Exp Med Biol. 2010; 703: 63–74. [DOI] [PubMed] [Google Scholar]
- 5. Sparrow JR,, Duncker T. Fundus Autofluorescence and RPE lipofuscin in age-related macular degeneration. J Clin Med. 2014; 3: 1302–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eldred GE,, Vitamins A. and E in RPE lipofuscin formation and implications for age-related macular degeneration. Prog Clin Biol Res. 1989; 314: 113–129. [PubMed] [Google Scholar]
- 7. Holz FG,, Schutt F,, Kopitz J,, et al. Inhibition of lysosomal degradative functions in RPE cells by a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 1999; 40: 737–743. [PubMed] [Google Scholar]
- 8. Burke TR,, Duncker T,, Woods RL,, et al. Quantitative fundus autofluorescence in recessive Stargardt disease. Invest Ophthalmol Vis Sci. 2014; 55: 2841–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duncker T,, Greenberg JP,, Ramachandran R,, et al. Quantitative fundus autofluorescence and optical coherence tomography in best vitelliform macular dystrophy. Invest Ophthalmol Vis Sci. 2014; 55: 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duncker T,, Tsang SH,, Lee W,, et al. Quantitative fundus autofluorescence distinguishes ABCA4-associated and non-ABCA4-associated bull's-eye maculopathy. Ophthalmology. 2014; 122: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duncker T,, Tsang SH,, Woods RL,, et al. Quantitative fundus autofluorescence and optical coherence tomography in PRPH2/RDS- and ABCA4-associated disease exhibiting phenotypic overlap. Invest Ophthalmol Vis Sci. 2015; 56: 3159–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lorenz B,, Wabbels B,, Wegscheider E,, Hamel CP,, Drexler W,, Preising MN. Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology. 2004; 111: 1585–1594. [DOI] [PubMed] [Google Scholar]
- 13. Kim SR,, Fishkin N,, Kong J,, Nakanishi K,, Allikmets R,, Sparrow JR. Rpe65 Leu450Met variant is associated with reduced levels of the retinal pigment epithelium lipofuscin fluorophores A2E and iso-A2E. Proc Natl Acad Sci U S A. 2004; 101: 11668–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Katz ML,, Drea CM,, Eldred GE,, Hess HH,, Robison WG,, Jr. Influence of early photoreceptor degeneration on lipofuscin in the retinal pigment epithelium. Exp Eye Res. 1986; 43: 561–573. [DOI] [PubMed] [Google Scholar]
- 15. Katz ML,, Redmond TM. Effect of Rpe65 knockout on accumulation of lipofuscin fluorophores in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2001; 42: 3023–3030. [PubMed] [Google Scholar]
- 16. Marsiglia M,, Lee W,, Mahajan VB,, et al. Quantitative autofluorescence as a clinical tool for expedited differential diagnosis of retinal degeneration. JAMA Ophthalmol. 2015; 133: 219–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ciardella AP,, Lee GC,, Langton K,, Sparrow J,, Chang S. Autofluorescence as a novel approach to diagnosing macular holes. Am J Ophthalmol. 2004; 137: 956–959. [DOI] [PubMed] [Google Scholar]
- 18. Delori F,, Greenberg JP,, Woods RL,, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2011; 52: 9379–9390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duncker T,, Lee W,, Tsang SH,, et al. Distinct characteristics of inferonasal fundus autofluorescence patterns in Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013; 54: 6820–6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duncker T,, Tabacaru MR,, Lee W,, Tsang SH,, Sparrow JR,, Greenstein VC. Comparison of near-infrared and short-wavelength autofluorescence in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013; 54: 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greenberg JP,, Duncker T,, Woods RL,, Smith RT,, Sparrow JR,, Delori FC. Quantitative fundus autofluorescence in healthy eyes. Invest Ophthalmol Vis Sci. 2013; 54: 5684–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sparrow JR,, Wu Y,, Nagasaki T,, Yoon KD,, Yamamoto K,, Zhou J. Fundus autofluorescence and the bisretinoids of retina. Photochem Photobiol Sci. 2010; 9: 1480–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Landrum JT,, Bone RA,, Moore LL,, Gomez CM. Analysis of zeaxanthin distribution within individual human retinas. Methods Enzymol. 1999; 299: 457–467. [DOI] [PubMed] [Google Scholar]
- 24. Kijlstra A,, Tian Y,, Kelly ER,, Berendschot TT. Lutein: more than just a filter for blue light. Prog Retin Eye Res. 2012; 31: 303–315. [DOI] [PubMed] [Google Scholar]
- 25. Bian Q,, Gao S,, Zhou J,, et al. Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Rad Biol Med. 2012; 53: 1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. SanGiovanni JP,, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005; 24: 87–138. [DOI] [PubMed] [Google Scholar]
- 27. Querques G,, Souied EH. The role of omega-3 and micronutrients in age-related macular degeneration. Surv Ophthalmol. 2014; 59: 532–539. [DOI] [PubMed] [Google Scholar]
- 28. Jeffrey BG,, Neuringer M. Age-related decline in rod phototransduction sensitivity in rhesus monkeys fed an n−3 fatty acid-deficient diet. Invest Ophthalmol Vis Sci. 2009; 50: 4360–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Neuringer M,, Connor WE,, Lin DS,, Barstad L,, Luck S. Biochemical and functional effects of prenatal and postnatal omega 3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci U S A. 1986; 83: 4021–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. SanGiovanni JP,, Parra-Cabrera S,, Colditz GA,, Berkey CS,, Dwyer JT. Meta-analysis of dietary essential fatty acids and long-chain polyunsaturated fatty acids as they relate to visual resolution acuity in healthy preterm infants. Pediatrics. 2000; 105: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 31. Mitchell DC,, Niu SL,, Litman BJ. Quantifying the differential effects of DHA and DPA on the early events in visual signal transduction. Chem Phys Lipids. 2012; 165: 393–400. [DOI] [PubMed] [Google Scholar]
- 32. Bazan NG,, Molina MF,, Gordon WC. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Ann Rev Nutr. 2011; 31: 321–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson EJ,, Chung HY,, Caldarella SM,, Snodderly DM. The influence of supplemental lutein and docosahexaenoic acid on serum lipoproteins, and macular pigmentation. Am J Clin Nutr. 2008; 87: 1521–1529. [DOI] [PubMed] [Google Scholar]
- 34. Delyfer MN,, Buaud B,, Korobelnik JF,, et al. Association of macular pigment density with plasma omega-3 fatty acids: the PIMAVOSA study. Invest Ophthalmol Vis Sci. 2012; 53: 1204–1210. [DOI] [PubMed] [Google Scholar]
- 35. Seddon JM,, Ajani UA,, Sperduto RD,, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Disease Case-Control Study Group. JAMA. 1994; 272: 1413–1420. [PubMed] [Google Scholar]
- 36. SanGiovanni JP,, Chew EY,, Clemons TE,, et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch Ophthalmol. 2007; 125: 671–679. [DOI] [PubMed] [Google Scholar]
- 37. Wu J,, Cho E,, Willett WC,, Sastry SM,, Schaumberg DA. Intakes of lutein zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015; 133: 1414–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. AREDS Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 39. Smith W, Mitchell P, Leeder SR. Dietary fat and fish intake and age-related maculopathy. Arch Ophthalmol. 2000; 118: 401–404. [DOI] [PubMed] [Google Scholar]
- 40. Seddon JM,, Rosner B,, Sperduto RD,, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001; 119: 1191–1199. [DOI] [PubMed] [Google Scholar]
- 41. SanGiovanni JP,, Chew EY,, Agron E,, et al. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008; 126: 1274–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merle BM,, Maubaret C,, Korobelnik JF,, et al. Association of HDL-related loci with age-related macular degeneration and plasma lutein and zeaxanthin: the Alienor study. PLoS One. 2013; 8: e79848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Reynolds R,, Rosner B,, Seddon JM. Dietary omega-3 fatty acids, other fat intake, genetic susceptibility, and progression to incident geographic atrophy. Ophthalmology. 2013; 120: 1020–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neuringer M,, Sandstrom MM,, Johnson EJ,, Snodderly DM. Nutritional manipulation of primate retinas, I: effects of lutein or zeaxanthin supplements on serum and macular pigment in xanthophyll-free rhesus monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 3234–3243. [DOI] [PubMed] [Google Scholar]
- 45. Leung IYF,, Sandstrom MM,, Zucker CL,, Neuringer M,, Snodderly DM. Nutritional manipulation of primate retinas II: Effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004; 45: 3244–3256. [DOI] [PubMed] [Google Scholar]
- 46. Barker FM,, 2nd,, Snodderly DM,, Johnson EJ,, et al. Nutritional manipulation of primate retinas, V: effects of lutein, zeaxanthin, and n-3 fatty acids on retinal sensitivity to blue-light-induced damage. Invest Ophthalmol Vis Sci. 2011; 52: 3934–3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jeffrey BG,, Mitchell DC,, Gibson RA. Neuringer M. n-3 fatty acid deficiency alters recovery of the rod photoresponse in rhesus monkeys. Invest Ophthalmol Vis Sci. 2002; 43: 2806–2814. [PubMed] [Google Scholar]
- 48. von Ruckmann A,, Fitzke FW,, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997; 38: 478–486. [PubMed] [Google Scholar]
- 49. Delori FC,, Goger DG,, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001; 42: 1855–1866. [PubMed] [Google Scholar]
- 50. Wing GL,, Blanchard GC,, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978; 17: 601–607. [PubMed] [Google Scholar]
- 51. Wikler KC,, Williams RW, Rakic P. Photoreceptor mosaic: number and distribution of rods and cones in the rhesus monkey retina. J Comp Neurol. 1990; 297: 499–508. [DOI] [PubMed] [Google Scholar]
- 52. Curcio CA,, Sloan KR,, Kalina RE,, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990; 292: 497–523. [DOI] [PubMed] [Google Scholar]
- 53. Ach T,, Huisingh C,, McGwin G,, Jr, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014; 55: 4832–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weiter JJ,, Delori FC,, Wing GL,, Fitch KA. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest Ophthalmol Vis Sci. 1986; 27: 145–152. [PubMed] [Google Scholar]
- 55. Sommerburg OG,, Siems WG,, Hurst JS,, Lewis JW,, Kliger DS,, van Kuijk FJ. Lutein and zeaxanthin are associated with photoreceptors in the human retina. Curr Eye Res. 1999; 19: 491–495. [DOI] [PubMed] [Google Scholar]
- 56. Rapp LM,, Maple SS,, Choi JH. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000; 41: 1200–1209. [PubMed] [Google Scholar]
- 57. Sparrow JR,, Gregory-Roberts E,, Yamamoto K,, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012; 31: 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Katz ML,, Stone WL,, Dratz EA. Fluorescent pigment accumulation in retinal pigment epithelium of antioxidant-deficient rats. Invest Ophthalmol Vis Sci. 1978; 17: 1049–1058. [PubMed] [Google Scholar]
- 59. Katz ML,, Parker KR,, Handelman GJ,, Bramel TL,, Dratz EA. Effects of antioxidant nutrient deficiency on the retina and retinal pigment epithelium of albino rats: a light and electron microscopic study. Exp Eye Res. 1982; 34: 339–369. [DOI] [PubMed] [Google Scholar]
- 60. Katz ML,, Drea CM,, Robison WG,, Jr. Relationship between dietary retinol and lipofuscin in the retinal pigment epithelium. Mech Ageing Dev. 1986; 35: 291–305. [DOI] [PubMed] [Google Scholar]
- 61. Katz ML,, Robison WG,, Jr. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch Gerontol Geriatr. 2002; 34: 169–184. [DOI] [PubMed] [Google Scholar]
- 62. Robison WG,, Jr, Kuwabara T,, Bieri JG. Deficiencies of vitamins E and A in the rat. Retinal damage and lipofuscin accumulation. Invest Ophthalmol Vis Sci. 1980; 19: 1030–1037. [PubMed] [Google Scholar]
- 63. Katz ML,, Stientjes HJ,, Gao CL,, Christianson JS. Iron-induced accumulation of lipofuscin-like fluorescent pigment in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1993; 34: 3161–3171. [PubMed] [Google Scholar]
- 64. Sundelin SP,, Nilsson SE. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Rad Biol Med. 2001; 31: 217–225. [DOI] [PubMed] [Google Scholar]
- 65. Nilsson SE,, Sundelin SP,, Wihlmark U,, Brunk UT. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol. 2003; 106: 13–16. [DOI] [PubMed] [Google Scholar]
- 66. Bhosale P,, Serban B,, Bernstein PS. Retinal carotenoids can attenuate formation of A2E in the retinal pigment epithelium. Arch Biochem Biophys. 2009; 483: 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sparrow JR,, Yoon KD,, Wu Y,, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010; 51: 4351–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sparrow JR,, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005; 80: 595–606. [DOI] [PubMed] [Google Scholar]
- 69. Kim SR,, Nakanishi K,, Itagaki Y,, Sparrow JR. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006; 82: 828–839. [DOI] [PubMed] [Google Scholar]
- 70. Dornstauder B,, Suh M,, Kuny S,, et al. Dietary docosahexaenoic acid supplementation prevents age-related functional losses and A2E accumulation in the retina. Invest Ophthalmol Vis Sci. 2012; 53: 2256–2265. [DOI] [PubMed] [Google Scholar]
- 71. Mukherjee PK,, Marcheselli VL,, Serhan CN,, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004; 101: 8491–8496. [DOI] [PMC free article] [PubMed] [Google Scholar]