Figure 1.
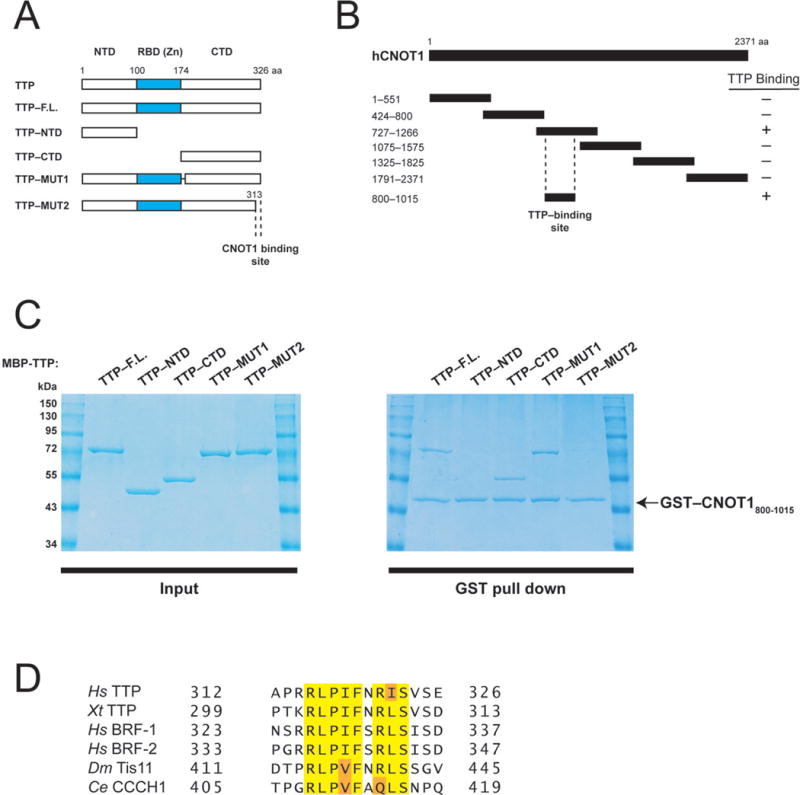
Human TTP directly binds a central fragment of CNOT1. (A) Schematic diagram of full-length TTP and TTP fragments used in co-precipitation experiments (Fig. 1C). Dashed lines indicate the region required for CNOT1 binding (Fig. 1C). (B) Schematic diagram of full-length CNOT1 and CNOT1 fragments used in co-precipitation experiments (Fig. 1C and Supplemental Fig. 1). Coordinates are marked to the left of each fragment. Dashed lines indicate the region required for TTP binding (Figure 1C). (C) Right panel: Recombinant glutathione-S-transferase (GST)–tagged CNOT1800–1015 (Figure 1B) was immobilized on glutathione-Sepharose beads and incubated with (MBP)–tagged full-length TTP, or TTP fragments (see left panel). Precipitated proteins were separated by SDS-PAGE and visualized by Coomassie staining. (D) Sequence alignment of conserved amino acids within the TTP C-terminus that binds CNOT1 of human (Hs) TTP, BRF-1, and BRF-2, Xenopus tropicalis (Xt) TTP, Drosophila melanogaster (Dm), Tis11 and Caenorhabditis elegans (Ce) CCCH1. Highlighted are amino acids identical in 100% of proteins (yellow) or conservative substitutions by related amino acids (orange).
