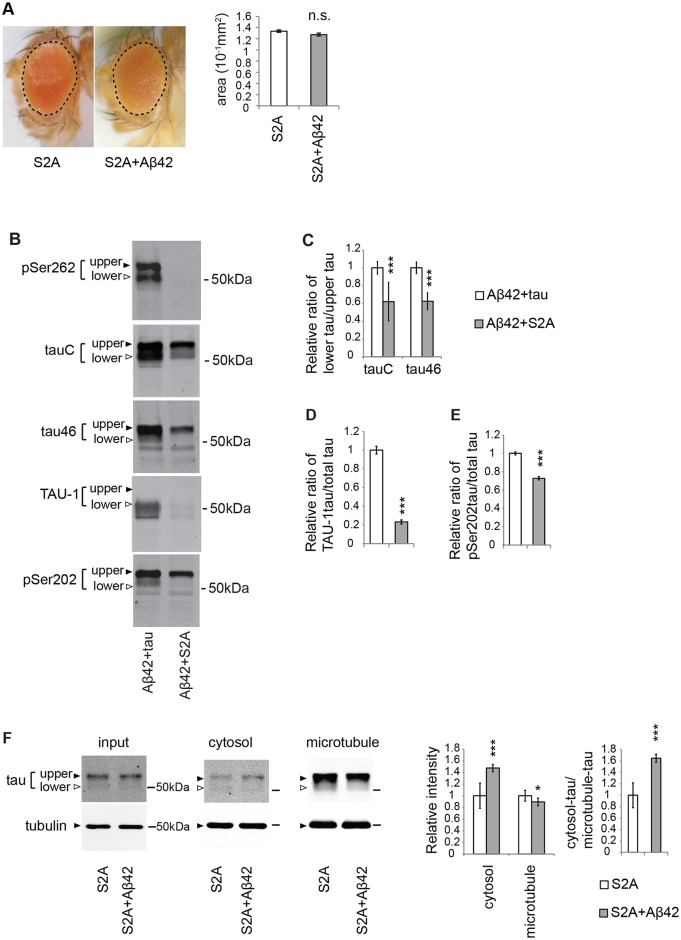
Fig 3. Blocking tau phosphorylation at Ser262/356 with unphosphorylatable alanine substitutions (S2A) preferentially reduces the levels of taulower and partially suppresses the Aβ42-mediated increase in tau mislocalization from the microtubule to the cytosol.
(A) Expression of either S2Atau (S2A) alone or co-expression of S2Atau and Aβ42 (S2A+Aβ42) does not cause eye degeneration. No significant difference in the surface area of the external eyes between S2A and S2A+ Aβ42 (mean ± SE, n = 6–8, N.S., not significant (p > 0.05) by one-way ANOVA). Transgene expression was driven by gmr-GAL4. (B) S2Atau shows different phosphorylation profiles compared to wild-type tau. Wild-type tau or S2Atau were co-expressed with Aβ42 (tau+Aβ42 and Aβ42+S2A, respectively) and subjected to western blotting with pan-tau antibody (tauC and tau46) or antibodies that recognize phosphorylation status of tau at the specific sites (pSer262, TAU-1 and pSer202). (C) The ratio of signal intensities of taulower to tauupper detected by tauC. (D) The ratio of signal intensities of TAU-1 blot to tauC blot. Mean ± SD, n = 5; ***, p < 0.005 by Student's t-test. (E) The ratio of signal intensities of pSer202 blot to tauC blot. Mean ± SD, n = 5; ***, p < 0.005 by Student's t-test. (F) Aβ42 increased the level of S2Atau in the cytosol and decreased those in the microtubule fraction, while the effects were smaller than those in wild-type tau. Mean ± SD, n = 4; *, p < 0.05, ***, p < 0.005 compared by Student's t-test. Representative blots are shown. Transgene expression was driven by gmr-GAL4.