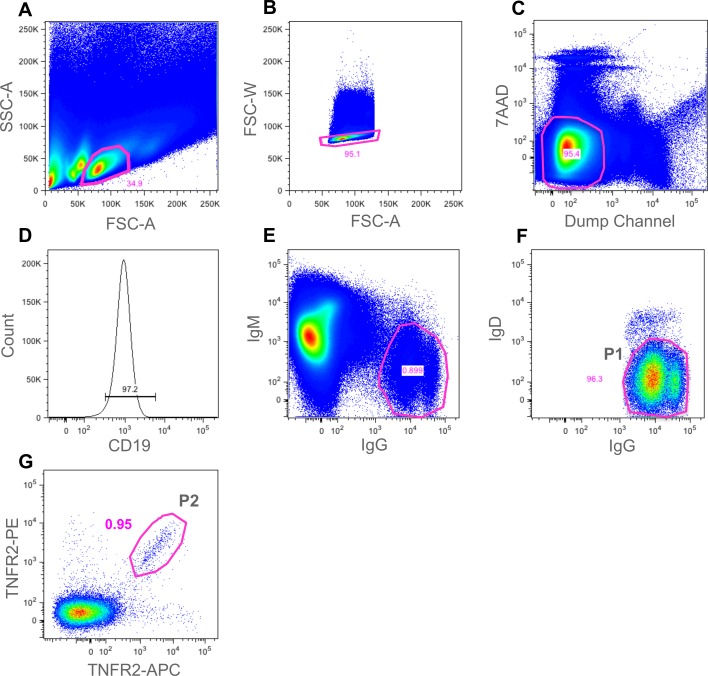
Fig 3. Gating strategy for identification of antigen-specific mouse memory B cells from TNFR2 immunised mice.
Following cell enrichment using CD45R microbeads (Miltenyi Biotech), cells were analysed in a BD FACS ARIA III. A gate was drawn around the lymphocyte population (gated population represented 34.9% of events) (A). FSC-W and FSC-A were then used to eliminate doublets (gated population represented 95.1% of events) (B). T cells, macrophages, neutrophils and 7AAD+ dead cells were eliminated in the “dump channel” (gated population represented 95.4% of events) (C). CD19+ B cells were identified (gated population represented 97.2% of events) (D). IgG+/IgM- B cells were then gated on (gated population represented 0.899% of events) (E). To further eliminate naïve B cells, IgD staining allowed gating for IgG+/IgD- cells (gated population (gate P1) represented 96.3% of events) (F). In order to demonstrate the importance of the antigen staining step, we sorted single cells from the total IgG+/IgD- cell population (gate P1). Finally, dual-colour antigen staining allowed a gate (P2) to be drawn around the double-positive antigen-specific population (gated population P2 represented 0.95%of events) (G). Single cells from gate P2 were sorted into a 96-well plate for single-cell RT-PCR.