Figure 2. The potential PP1α-interacting KVFF motif of AR is not essential for AR-PP1α interaction.
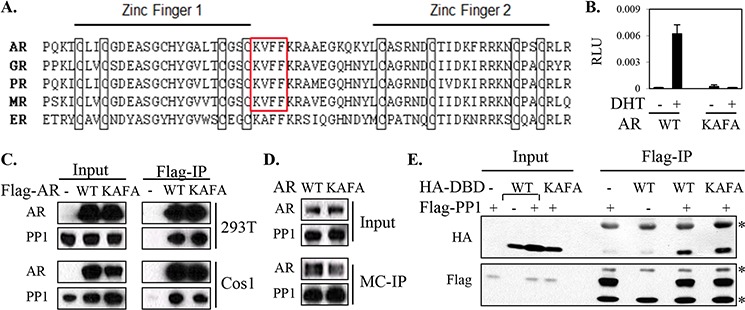
A. A schematic drawing shows the linear amino acid sequence alignment of the zinc finger regions in the DNA binding domains of the class-I steroid receptors. Highlighted in the frame is the unique PP1α-interacting consensus motif (KVxF) that exists in AR, GR, PR, and MR, but not ERα. Amino acid sequences are based on human androgen receptor (AR; GenBank M20132.1); human glucocorticoid receptor (GRα; NM_001018077); human progesterone receptor (PR; M15716.1); human mineralocorticoid receptor (MR, NM_000901.3); and human estrogen receptor (ERα; NM_000125.3). B. AR-deficient PCa cell line (PC3) was transfected with AR-mediated probasin-Luc and CMV-Renilla reporters, together with AR wild-type (WT) versus KVFF mutant (KVFF to KAFA). Cells were then incubated for overnight in androgen-depleted medium with 10 nM of DHT for Dual-Luc analysis. C. 293T and Cos1 cells were co-transfected with PP1α and Flag-tagged AR wild-type versus KVFF mutant (KAFA), with the empty Flag vector (−) as control. Co-immunoprecipitation (Co-IP) assay was carried out using anti-Flag-M2 beads. D. HeLa cells transfected with AR wild-type versus KAFA mutant were incubated for overnight in androgen-depleted medium with 10 nM of DHT, followed by microcystin (MC)-IP and blotting. E. Flag-tagged PP1α was co-transfected in 293T cells with HA tagged AR-DBD wild-type (WT) versus DBD mutant (KAFA) constructs, followed by Co-IP using anti-Flag-M2 beads and blotting. Flag or HA empty vectors (−) were used as controls, respectively. Non-specific IgG bands are marked (*).
