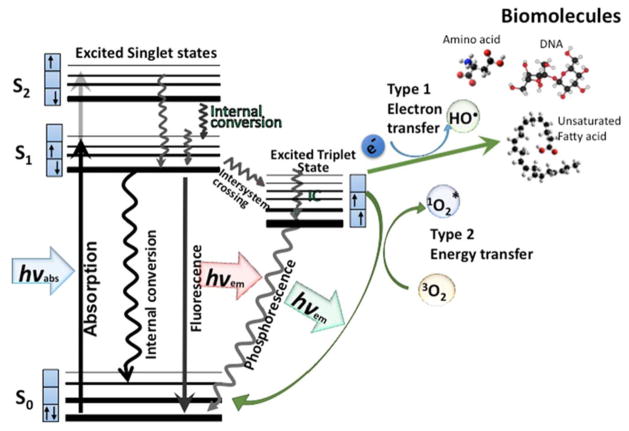
Figure 1. Jablonski diagram.
When light (hv) is absorbed by the PS, the electron moves from a non-excited low-energy singlet state into a high-energy singlet state. This excited state can lose energy by emitting a photon (fluorescence) or by internal conversion (non-radiative decay). The process known as intersystem crossing involves flipping of the spin of the high-energy electron, leading to a long-lived excited triplet state. In the presence of molecular oxygen, superoxide and hydroxyl radicals are formed in Type I reactions and singlet oxygen in a Type II reaction. These ROS can damage most types of biomolecules (amino acids, lipids, nucleic acids).