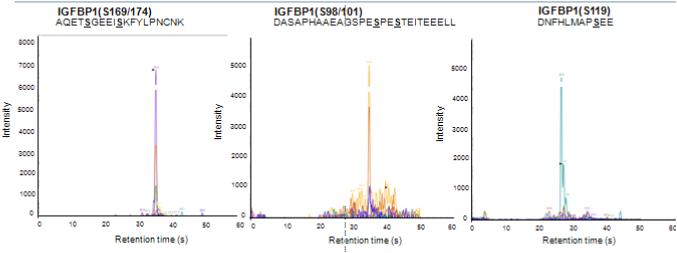
Figure 1. MRM spectra of synthetic IGFBP-1 peptides used for CK2 in vitro kinase assay.
MRM/MS analysis of phosphorylation of Ser residues (S) on IGFBP-1 peptides, non-phosphorylated synthetic peptides were used as substrates for CK2 enzyme. Following incubation, CK2-induced phosphorylation of each peptide was then monitored by targeting the phosphorylation-specific version of each IGFBP-1 peptide substrate by MRM-MS using a QTRAP 4000 mass spectrometer. CK2 phosphorylates IGFBP-1 at Ser101, Ser119 and Ser169. Ser101 was found to be doubly phosphorylated with Ser98, and Ser169 was indicated to be doubly phosphorylated with Ser174.