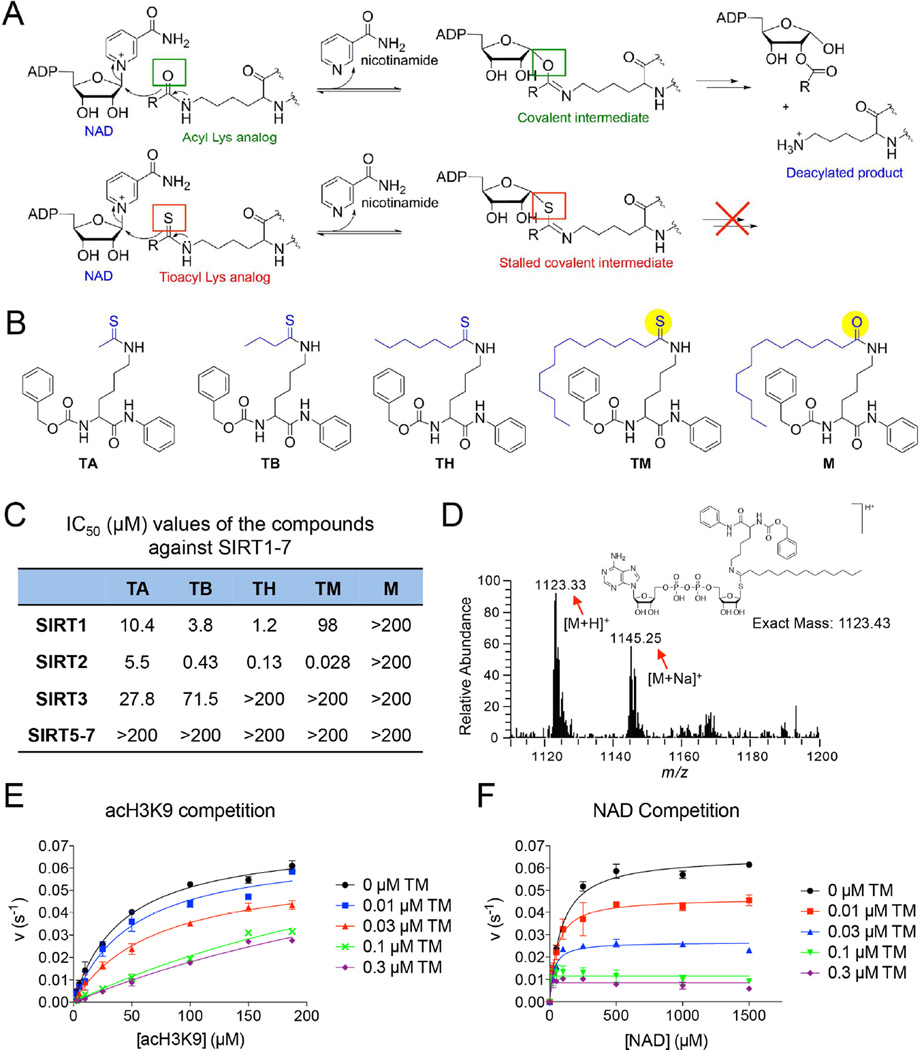
Figure 1.
Development of mechanism-based inhibitor of sirtuins. (A) The enzymatic reaction mechanism of sirtuin-catalyzed NAD-dependent deacylation (upper panel). Thioacyl lysine compounds act as suicide substrates to inhibit sirtuins (lower panel). (B) Structures of four different thioacyl lysine sirtuin inhibitors, TA, TB, TH, and TM. M, which differs from TM by just one atom (highlighted by yellow color), is an inactive control of TM. (C) IC50 (µM) values of the TA, TB, TH, TM and M against SIRT1–7. IC50 values derived from Graphpad Prism are presented as mean values from three independent experiments. (D) Mass spectrometry detection of the stable covalent intermediate formed by TM and NAD. (E, F) Henri-Michaelie-Menten plots showing acH3K9 (E) and NAD (F) competition analyses of TM-mediated SIRT2 inhibition. Error bars represent mean ± sd. See also Figure S1.