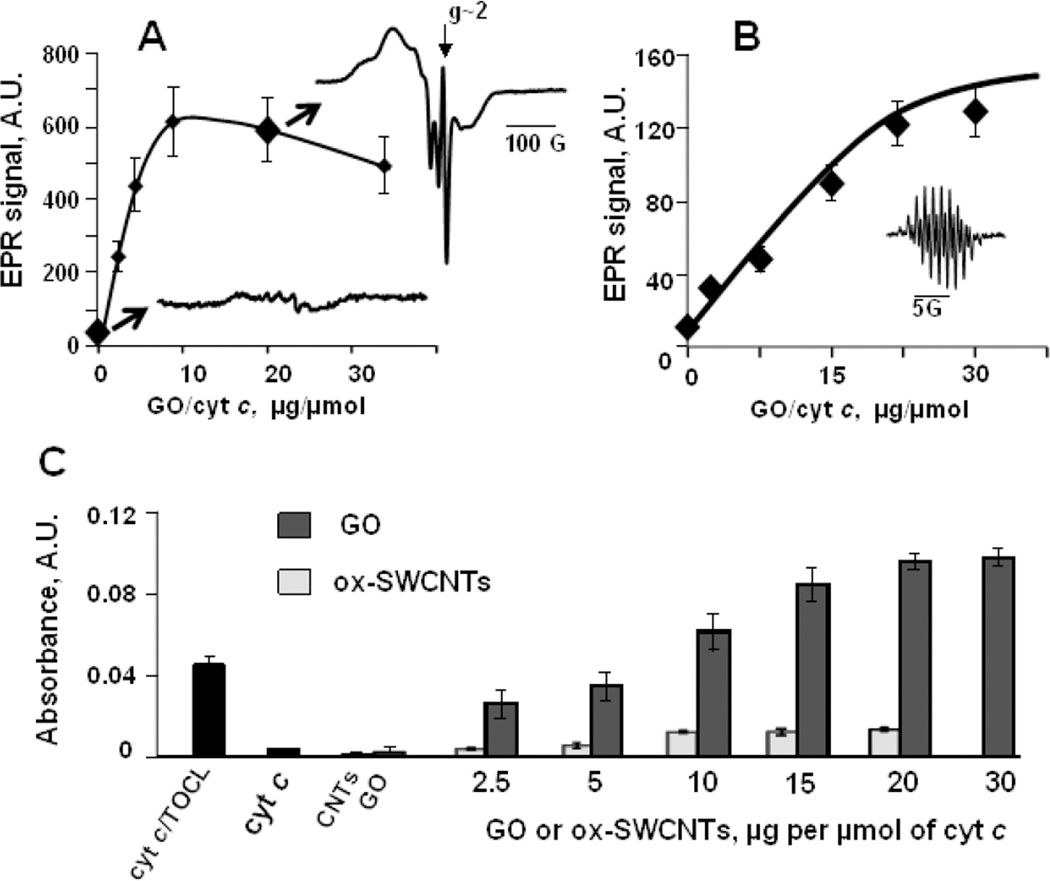
Fig. 5. Binding of GO to cyt c causes unfolding of the protein and increases the accessibility of cyt c active site.
A) - GO facilitates cyt c nitrosylation as evidenced by low temperature EPR spectroscopy. Magnitude of the signal of nitrosylated cyt c depends on GO/cyt c ratio revealing the increase of the accessibility of cyt c active site towards nitric oxide upon enzyme binding to GO (Vlasova et al., 2006). Inserts are EPR spectra of cyt c nitrosylated in the absence (blue) and in the presence (red) of GO. EPR spectrum of nitrosylated cyt c/GO complexes includes pronounced narrow GO spectrum at g~2. Reduced cyt c (100 µM, 500 µM ascorbate) was incubated with GO at 37°C for 5 min in 50 mM sodium phosphate buffer, pH 7.4, containing 100 µM DTPA, then 750 µM PAPANONOate was added and incubation was continued for additional 15 min. The reaction was stopped by freezing the samples in liquid nitrogen. EPR spectra of nitrosylated cyt c were measured at 77K.
Binding to GO confers peroxidase activity on cyt c: B) - Magnitude of EPR spectrum of etoposide-phenoxyl radicals (insert) generated in the peroxidase reaction of cyt c/GO complexes in 2 min after addition of 100 µM H2O2 (7.5 µM cyt c) (Kagan et al., 1999); C) - Oxidation of peroxidase substrate ABTS by cyt c/GO. Oxidation of ABTS to yield the product with absorbance maximum at 410 nm was performed at reagent concentrations: 5 µM cyt c, 1 mM ABTS and 100 µM H2O2. The effect of GO on cyt c peroxidase activity is compared to the effects of TOCL (blue bar) and ox-SWCNTs.