Introduction
The term “Carcinosarcoma” describes a tumor composed of malignant epithelial and mesenchymal elements. Various organs commonly affected include uterus, lung, oesophagus, pancreas and kidney, with gall bladder being the unusual site of involvement [1]. Gall bladder carcinoma (GBC), a relatively rare disease in western world, is one of the most common hepato-biliary neoplasm diagnosed in north Indian Gangetic planes. Most common histological type of GBC is adenocarcinoma (80–95 %). Less commonly described histological variants of GBC are undifferentiated/anaplastic carcinoma (2–7 %), squamous cell carcinoma (1–6 %), adenosquamous (1–4 %) and small cell type (1–3 %); carcinosarcoma is a rarely described subtype of GBC. The first case of carcinosarcoma of gall bladder [CSGB] was described by Karl Landsteiner in 1907 [2]. Till date, about 80 cases have been reported in the literature with a mean tumor size 8.4 ± 3.7 cm (range, 2.5–16 cm) [3]. We report here a case of large CSGB (35 × 25 × 20 cm) successfully treated by surgical resection. After an exhaustive literature review, we can say that it is probably the largest being reported till date in the literature.
Case Report
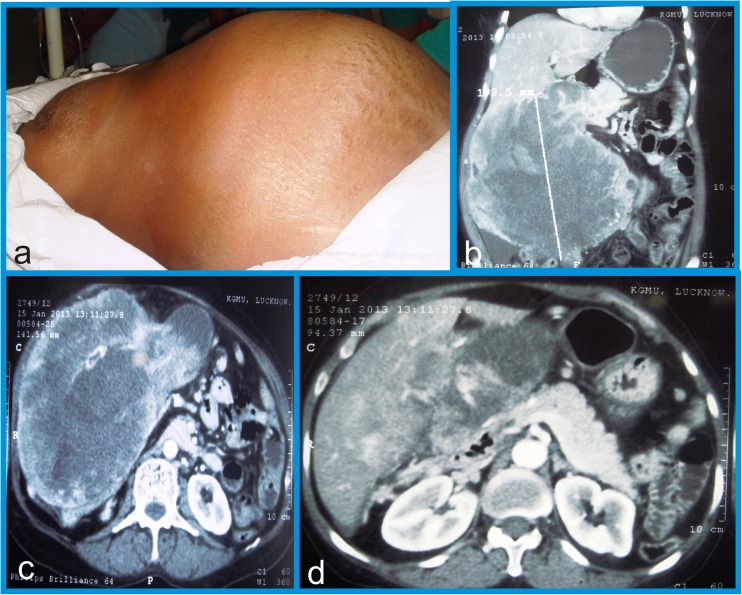
A 46 year old poorly nourished woman presented in our clinic with a 9 months history of constant dull aching pain and gradually progressive mass in the right hypochondrium. Abdominal examination revealed large (measuring 24 × 25 cm), mildly tender, globular gall bladder mass causing a distinct bulge in right hypochondrium (Fig. 1a). Physical examination showed no jaundice, ascites or supraclavicular lymphadenopathy. Laboratory investigations revealed normal hematological parameters. Liver function tests revealed normal serum bilirubin but raised serum alkaline phosphatase (642 U/L, normal- 42–129 U/L).
Fig. 1.
a Abdominal examination demonstrating large palpable globular gall bladder mass (24 × 25cm) in right hypochondrium. b, c & d Computed tomography revealed exophytic heterogeneous soft tissue mass (22.4 × 19.2 × 14.3 cm) arising from fundus and body of gall bladder with infiltration of adjacent liver parenchyma
Contrast-enhanced computed tomography (CECT) of the abdomen revealed large well defined, exophytic heterogeneous soft tissue mass (22.4 × 19.2 × 14.3 cm) arising from fundus and body of gall bladder, infiltrating adjacent segment 4b of liver. Tumor was extending from right hypochondrium to right iliac fossa, with ill-defined interface with duodenum (D2) and hepatic flexure of colon. There was no demonstrable common bile duct/intra-hepatic biliary ductal dilatation, ascites and no clinically significant loco-regional lymphadenopathy. There was no evident portal vein / hepatic artery compression or infiltration (Fig. 1b, c & d). There was no evidence of distant metastasis. Tumor markers (Carcinoembryonic antigen (CEA), CA19-9 and Alpha fetoprotein (AFP)) were all within normal limits. Ultrasound guided Fine needle aspiration cytology (FNAC) of gall bladder mass revealed poorly differentiated adenocarcinoma. Chest X-ray was negative.
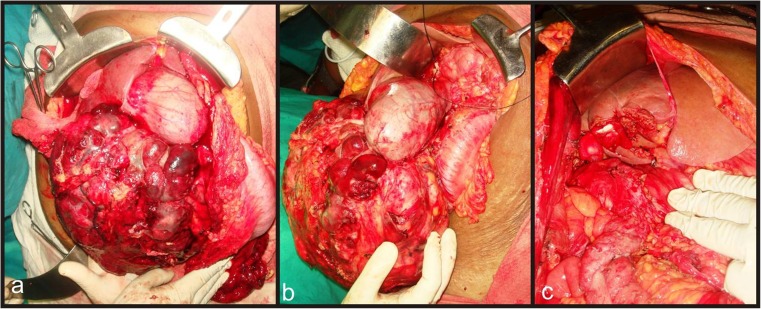
After excluding major vascular involvement, non-contiguous liver infiltration and clinically significant lymphadenopathy and with preoperative diagnosis of locally advanced gallbladder carcinoma, the patient was planned for exploratory laparotomy. Intra-operatively, large bosselated, highly vascular, soft tissue mass was found arising mainly from fundus and body of the gallbladder with limited infiltration of adjacent liver parenchyma (segment 4b and 5) Fig. 2a & b), abutting transverse colon and duodenum. No ascites, loco-regional lymphadenopathy, omental / mesenteric deposits or distant metastasis noted. Patient underwent Radical cholecystectomy with hepato-duodenal ligament lymph node clearance and segment 4b/5 liver resection in order to achieve curative (R0) resection Fig. 2c).
Fig. 2.
a & b Laparotomy revealed large lobulated soft tissue mass arising from fundus & body of gall bladder with involvement of adjacent hepatic parenchyma (blue arrow- gall bladder mass, black arrow- reflected hepatic flexure of colon). c View of the surgical field post surgical resection of gall bladder mass
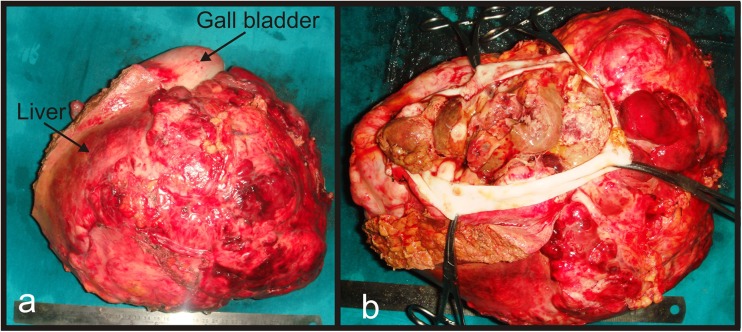
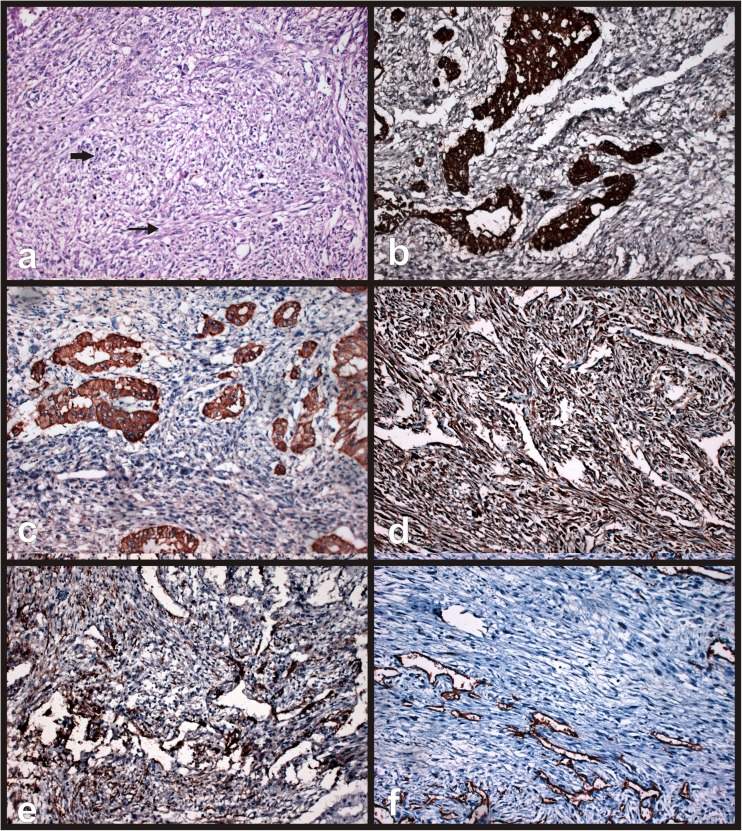
Pathological findings on gross examination revealed lobulated soft tissue mass measuring 35 × 25 × 20cm involving fundus and body of gall bladder and infiltrating into adjacent liver parenchyma with no coexistent gall stones (Fig. 3a, b & c). Resection margin of gall bladder and liver bed were free of tumor cells. Five nodes harvested were all reactive in nature. Histologically, tumor showed biphasic malignant elements comprising of both epithelial and mesenchymal components. Epithelial component was composed predominantly of acini lined by columnar - cuboidal cells with high nucleo-cytoplasmic ratio and pleomorphic hyper chromatic nuclei. Mesenchymal component showed fusiform shaped spindle cells with scanty cytoplasm (Fig. 4a). No evidence of vascular permeation, perineural invasion or lymphatic permeation was seen.
Fig. 3.
a Gross examination showed tumor was arising from fundus of gall bladder and measuring 35 × 25 cm. b Cut open specimen of gall bladder showing polypoidal growth arising from posterior wall of gall bladder
Fig. 4.
a Microscopic examination revealed biphasic elements showing both epithelial (thick arrow) & sarcomatous component (thin arrow). b and c Immunohistochemical analysis revealed epithelial elements showing strong positivity for Cytokeratin (b) and focal positivity for Epithelial membrane antigen (c). d and e Sarcomatous elements showed strong positivity for Vimentin (d) and focal positivity for smooth muscle antigen (SMA) (e). f negative staining for CD34
Immunohistochemical studies revealed epithelial element of tumor cells strongly positive for cytokeratin (CK) (Fig. 4b), focal positivity for epithelial membrane antigen (EMA) (Fig. 4c), while mesenchymal component showed strong positivity for vimentin (Fig. 4d), focal positivity for smooth muscle actin (SMA) (Fig. 4e) and negativity for desmin, S-100 and CD 34 (Fig. 4f). Postoperative course was uneventful. Patient received adjuvant chemotherapy (6 cycles of cisplatin and doxorubicin). No recurrent lesion was found using abdominal ultrasound examination and CECT scan at 15 months after completion of treatment.
Discussion
Carcinosarcoma comprises atypical subset of gall bladder malignancies, representing less than 1 % of gall bladder neoplasms [1]. Carcinosarcoma is postulated to arise from totipotent stromal stem cells or embryonic cell rest and is characterized by presence of both epithelial and mesenchymal elements. Epithelial component usually consists of foci of adenocarcinoma and in rare instances squamous cell, small cell and undifferentiated cell carcinoma. Presence of squamous elements portend poor prognosis in view of rapid growth rate of tumor (2 times more) as compared to adenocarcinoma [3]. Most common mesenchymal component comprised of spindle cells and less frequently elements of cartilage, bone and other tissues [4]. Exact aetiopathogenesis of carcinosarcoma of gall bladder is not clearly understood with no well defined predisposing factors. No convincing association can be elucidated between gall stones and the incidence of CSGB.
CSGB occurs predominantly in elderly woman with a mean age of presentation 67.7 years (range 45 to 90 years) [3]. Most common presenting symptoms are vague abdominal pain, palpable abdominal lump and weight loss; jaundice is rarely a presenting symptom. In stark comparison to GBC, patients with CSGB usually present when tumor has grown to large size, with average tumor size reported being 8.4 ± 3.7cm (range, 2.5–16cm), [3]. CSGB is not associated with characteristic radiological findings other than disproportionately large tumor size with minimal adjacent organ infiltration and absence of significant loco-regional lymphadenopathy [5]. As it is not characterized by specific tumor marker elevation, conclusive preoperative diagnosis is difficult. CSGB is best treated with definitive surgical resection wherever possible. Surgical treatment includes simple/radical cholecystectomy, with or without adjacent local organ resection with aim to achieve margin negative resection. Overall 5 years survival after definitive surgery is 31 % [6].
Final diagnosis of CSGB is made postoperatively based on histopathological examination of biopsy tissue and immunohistochemical analysis. Immunohistochemistry (IHC) study reveals carcinomatous component staining positive for cytokeratin (CK) and epithelial membrane antigen (EMA), while sarcomatoid element staining strongly/focal positive for vimentin, smooth muscle actin (SMA) and desmin [7, 8].
Role of adjuvant chemotherapy in CSGB is debatable. It may have a role in inoperable/metastatic setting with minimal to moderate efficacy. Various anecdotal reports describe use of several chemotherapeutic agents including cisplatin, doxorubicin, 5-fluorouracil, ifosfamide and etoposide with variable response rates. Role of radiotherapy is equally debatable [9, 10].
Diagnosis of CSGB should be considered in differential diagnosis of the GBC when patients present with unusually large gall bladder tumor, with no accompanying adjacent organ involvement/ loco regional lymphadenopathy. Most important prognostic factors determining CSGB prognosis include tumor size, stage at presentation and feasibility of R0 resection [11].
Conclusion
CSGB is rare condition with universally poor prognosis. It is difficult to differentiate it from GBC based on clinical presentation and radiological investigation. CSGB should be considered as a differential diagnosis, especially when patients present with a large gall bladder mass with minimal adjacent organ infiltration or loco regional lymphadenopathy. Diagnosis is usually made postoperatively based on histological findings characterized by presence of both carcinomatous and sarcomatoid elements. Margin negative resection is the mainstay of treatment. Role of adjuvant chemo-radiotherapy is debatable.
Acknowledgments
Our sincere thanks to Prof Ravi Kant, Vice chancellor, King George’s Medical University, for his guidance and mentorship.
Compliance with Ethical Standards
We, the authors (Sameer Gupta, Chanabasappa Kori and Vijay Kumar), hereby certify that :
• This manuscript has not been submitted to more than one journal for simultaneous consideration, neither manuscript has been published previously (partly or in full)
• No data have been fabricated or manipulated (including images) to support our conclusions
• No plagiarism or violation of Copyright rules
• Consent to submit has been received explicitly from all co-authors, as well as from the responsible authorities - tacitly or explicitly - at the institute/organization. All Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
• No potential conflict of interest relevant to this article was reported
Conflict of Interest
Sameer Gupta, Chanabasappa Kori and Vijay Kumar, hereby declare that we have no conflict of interest.
Contributed by
Contributions SG - manuscript preparation, concept and design, VK-manuscript final approval; CK- manuscript preparation, data acquisition and analysis.
Abbreviations
- GBC
Gall bladder carcinoma
- CSGB
Carcinosarcoma of gall bladder
- FNAC
Fine needle aspiration cytology
- IHC
Immunohistochemistry
- CK
Cytokeratin
- EMA
Epithelial membrane antigen
- SMA
Smooth muscle antigen
- CD
Cluster of differentiation
References
- 1.Baillie J. Tumors of the gallbladder and bile ducts. J Clin Gastroenterol. 1999;29:14–21. doi: 10.1097/00004836-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Landsteiner K. Plattenepithelkarzinom und sarkom der gallenblase in einem falle von cholelithiasis. Z Klin Med. 1907;62:427–433. [Google Scholar]
- 3.Okabayashi T, Sun ZL, Montogomery RA, Hanazaki K. Surgical outcome of carcinosarcoma of the gall bladder: a review. World J Gastroenterol. 2009;15:4877–4882. doi: 10.3748/wjg.15.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huguet KL, Hughes CB, Hewitt WR. Gallbladder carcinosarcoma: a case report and literature review. J Gastrointest Surg. 2005;9:818–821. doi: 10.1016/j.gassur.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Okabayashi T, Sun ZL, Montgomey RA, Hanazaki K. Surgical outcome of carcinosarcoma of the gall bladder: review. World J Gastroenterol. 2009;15:4877–4882. doi: 10.3748/wjg.15.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HH, Hur YH, Jeong EH, Park EK, Koh YS, Kim JC, Kim HJ, Kim JW, Cho CK. Carcinosarcoma of the gallbladder: report of two cases. Surg Today. 2012;42(7):670–675. doi: 10.1007/s00595-012-0160-6. [DOI] [PubMed] [Google Scholar]
- 7.Nishihara K, Tsuneyoshi M. Undifferentiated spindle cell carcinoma of the gallbladder: a clinicopathologic, immunohistochemical, and flow cytometric study of 11 cases. Hum Pathol. 1993;24:1298–1305. doi: 10.1016/0046-8177(93)90263-G. [DOI] [PubMed] [Google Scholar]
- 8.Li BF, Li PW, Shi R, Liu ZL, Liu YF. Clinicopathologic analysis of gallbladder carcinosarcoma. Am Surg. 2013;79(1):E37–E39. [PubMed] [Google Scholar]
- 9.Khanna M, Khanna A, Manjari M. Carcinosarcoma of the gallbladder: a case report and review of the literature. J Clin Diagn Res. 2013;7(3):560–562. doi: 10.7860/JCDR/2013/4924.2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotta T, Tanimura H, Yokoyama S, Ura K, Yamaue H. So-called carcinosarcoma of the Gall bladder; spindle cell carcinoma of the gallbladder: report of a case. Surg Today. 2002;32:462–467. doi: 10.1007/s005950200077. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Chen Z, Fukuma M, Lee LY, Wu M. Prognostic significance of race and tumor size in carcinosarcoma of gall bladder: a metaanalysis of 68 cases. Int J Clin Exp Pathol. 2008;1:75–83. [PMC free article] [PubMed] [Google Scholar]