Figure 2.
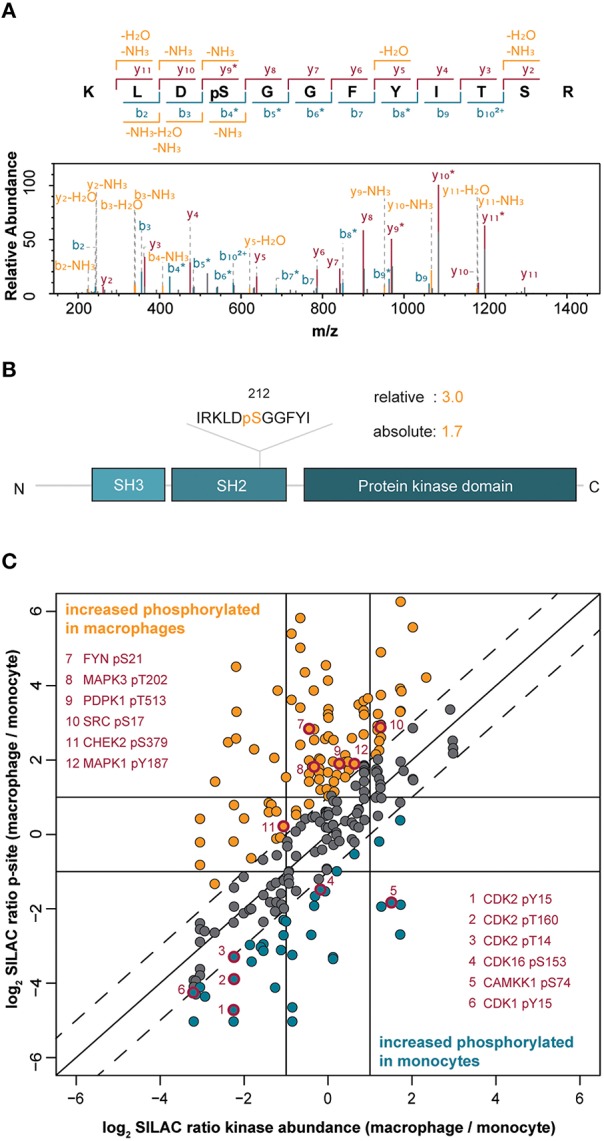
(A) Annotated MS/MS spectrum of the peptide from the tyrosine kinase Src spanning amino acids 209–220 with phosphorylation at Ser212. (B) Protein domain organization of Src indicating the pSer212 position in the SH2 domain. The difference in the level of phosphorylation of Ser212 between macrophage-like vs. monocytic THP-1 cells is indicated (relative change) and normalized to changes in the Src protein amount (absolute change). (C) Correlation of quantified phosphorylation sites with corresponding protein kinase abundance. The solid diagonal line indicates perfect correlation between changes in the amount of phosphorylations and changes in the corresponding protein kinase amounts, i.e., differences in phosphorylations are only attributed to changes in the protein amounts. The dashed lines indicate two-fold changes of phosphorylations (in both directions), i.e., changes in phosphorylations are not exclusively attributed to changes in protein abundance. Dots highlighted in orange indicate increased phosphorylation in macrophages whereas blue dots indicate increased phosphorylation in monocytes. Red-rimmed dots highlight kinase phosphorylation sites implicated in the induction of kinase activity.
