FIGURE 3.
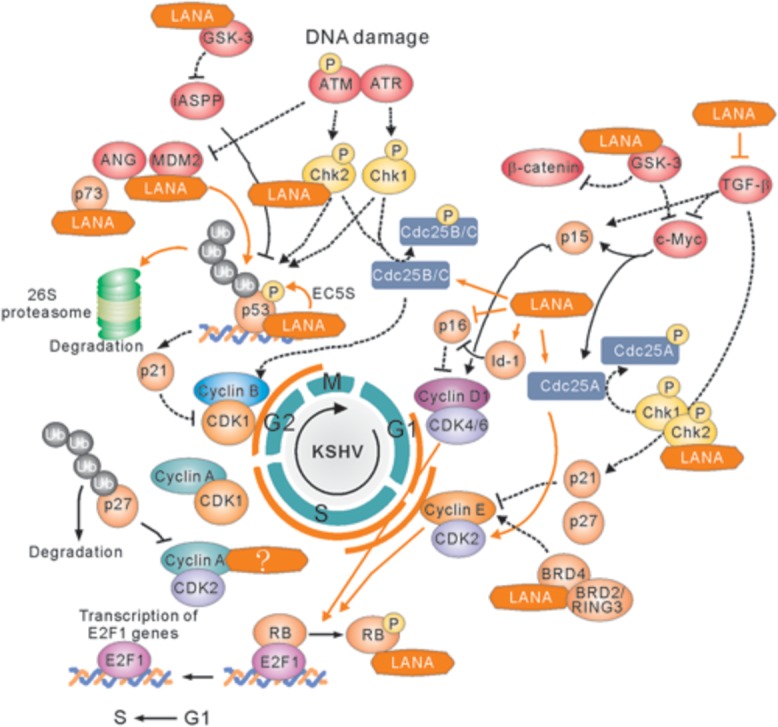
Latency-associated nuclear antigen-mediated deregulation of cell cycle. LANA associates with many cellular proteins including GSK-3, c-Myc, Chk2, BRD4, and BRD2/RING3, to activate Cyclin D1-CDK4/6 and Cyclin E-CDK2 complex, which results in the hyperphosphorylation form of retinoblastoma protein (RB). Hypersphophorylation of RB prevents its interaction with E2F, and releases E2F to activate expression of genes required for entry into the S phase. LANA also interacts with RB to facilitate G1/S phase transition. On the other hand, LANA not only recruits E3-ligase complex (including Elongin BC-Cullin 5, EC5S) or MDM2 to target p53 for ubiquitin-mediated degradation, but also associates with many p53-associated pathway regulators including p73, iASPP, and Chk2 to induce Cyclin B-CDK1 complex for driving G2/M phase transition. The positive and negative regulation by LANA is shown by solid orange line. The cellular signaling pathway blocked by LANA is indicated by dot line.
